Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human - PubMed (original) (raw)
. 2012 Jul 20;150(2):366-76.
doi: 10.1016/j.cell.2012.05.016. Epub 2012 Jul 12.
Pontus Boström, Lauren M Sparks, Li Ye, Jang Hyun Choi, An-Hoa Giang, Melin Khandekar, Kirsi A Virtanen, Pirjo Nuutila, Gert Schaart, Kexin Huang, Hua Tu, Wouter D van Marken Lichtenbelt, Joris Hoeks, Sven Enerbäck, Patrick Schrauwen, Bruce M Spiegelman
Affiliations
- PMID: 22796012
- PMCID: PMC3402601
- DOI: 10.1016/j.cell.2012.05.016
Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human
Jun Wu et al. Cell. 2012.
Abstract
Brown fat generates heat via the mitochondrial uncoupling protein UCP1, defending against hypothermia and obesity. Recent data suggest that there are two distinct types of brown fat: classical brown fat derived from a myf-5 cellular lineage and UCP1-positive cells that emerge in white fat from a non-myf-5 lineage. Here, we report the isolation of "beige" cells from murine white fat depots. Beige cells resemble white fat cells in having extremely low basal expression of UCP1, but, like classical brown fat, they respond to cyclic AMP stimulation with high UCP1 expression and respiration rates. Beige cells have a gene expression pattern distinct from either white or brown fat and are preferentially sensitive to the polypeptide hormone irisin. Finally, we provide evidence that previously identified brown fat deposits in adult humans are composed of beige adipocytes. These data provide a foundation for studying this mammalian cell type with therapeutic potential. PAPERCLIP:
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Figure 1. Beige fat cells arise from a subset of preadipocytes in the subcutaneous adipose depot
(A) Multilocular beige fat cells are prominent in the subcutaneous white adipose depot of 129SVE male mice at 7–9 weeks of age. Hematoxylin & Eosin stain show islets of multilocular fat cells within inguinal white fat depot (400X). (B) Total RNA was isolated from epididymal (Epi), inguinal (Ing) and interscapular (Bat) adipose tissues of 129SVE mice and assayed for mRNA expression for Ucp-1 and other brown fat-like genes by qPCR. Values are mean±SD (n=5). Expression levels of Ucp-1 and Cidea are undetected in epididymal depot. (C) Total RNA was isolated from fat cells differentiated from epididymal (Epi), inguinal (Ing) and interscapular (Bat) primary stromal vascular cells and assayed for mRNA expression for Ucp-1 and other brown fat-like genes by qPCR. Relative gene expression was normalized to adipsin mRNA levels. Values are mean±SD (n=3) (D) Cluster dendrogram of array expression data with RNA samples from differentiated cultures of 26 immortalized fat cell lines as described in text. Analysis details were described in the Supplemental Experimental Procedures. Height (y axis) is Euclidean distance. To mimic the sympathetic tone in vivo upon prolonged cold exposure when beige fat cells are functionally activated, we analyzed RNA from fully differentiated cells (day8), treated with 10 μM forskolin (FSK), a cAMP inducing agent, for 4 hrs.
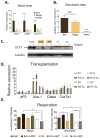
Figure 2. Beige fat cells show comparable thermogenic potential to brown fat cells upon stimulation
Representative lines from each group were further analyzed functionally. Bat1, bat6, bat8 for brown fat cells; X9, X3, D16, D12 for beige fat cells and N13, X7, J6, G18 for white fat cells. All these lines presented similar levels of adipogenesis and fat cell specific markers were measured via qPCR. See Figure S2A. Similar results were observed in more than three independent experiments. (A) Total RNA was isolated from the differentiated brown, beige and white clonal cell lines and was assayed for mRNA levels of brown fat-like genes (Ucp-1, Cidea and Cox7a1) by qPCR. (B) Total RNA was isolated from the differentiated brown, beige and white clonal cell lines treated with 10μM forskolin for 4 hours and was assayed for mRNA levels of Ucp-1. (C) Protein lysates were isolated from the differentiated brown, beige and white clonal lines either unstimulated or treated with 10 μM isoproterenol for 6 hours, and probed by immunoblot as indicated, with tubulin as a loading control. (D) Cell transplantations were done in the preadipose state as described in Experimental Procedures. After 6 weeks, fat pads were harvested and mRNA expression was analyzed by qPCR (n=5–8). For acute sympathetic stimulation, CL316,243, at 1mg kg−1, was injected intraperitoneally 5 hours before harvesting the transplanted fat pads. (E) Oxygen consumption in cultured brown, beige and white fat cells was assayed as described in Experimental Procedures. Oligomycin (ATP synthase inhibitor) was added to cells to measure the uncoupled respiration rate. The cells were treated with dibutyryl-cAMP for 12 hours before harvesting. Values are mean±SD (n=4). *, p<0.05; **, p<0.01; ***, p<0.001.
Figure 3. Beige and brown fat cells exhibit distinct gene expression profiles
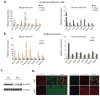
(A) Genes with a beige- or brown-selective expression pattern were first identified by microarray analysis as described in Supplemental Experimental Procedures. Relative mRNA expression for these genes was then measured by qPCR in differentiated brown, beige and white fat cells in an unstimulated state. (B) Analysis of beige- and brown-selective genes expression in adipose tissues isolated from inguinal WAT and interscapular BAT of 129 SVE mice. Values are mean±SD (n=5). *, p<0.05; **, p<0.01; ***, p<0.001. (C) Protein lysates of adipose tissues isolated from inguinal WAT and interscapular BAT of 129 SVE mice were probed with antibodies by immunoblot as indicated. Asterisk represents a nonspecific band as a loading control. (D) Microscopic and confocol images of mouse inguinal white and interscapular brown adipose tissue stained with antibodies recognizing CD137 (red) (microscopic images), TMEM26 (green) (confocal images) and UCP1 (green and red), with nuclei co-stained with DAPI as indicated. To induce the “browning” morphology and UCP1 expression in inguinal white adipose depot, 129SVE mice were injected intraperitoneally with CL316, 243, at 1mg kg−1 daily for 4 days. Inguinal and interscapular adipose tissues were harvested on the fifth day.
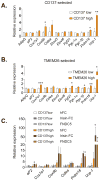
Figure 4. Isolation of beige precursor cells from inguinal stroma with beige-selective cell surface markers
Total RNA was isolated from differentiated cultures of beige-selective marker CD137 (A) and TMEM26 (B) positive and negative cells purified from inguinal SV cells; these adipocytes were then assayed for mRNA levels of general adipogenic markers, Ucp-1 and other characteristic thermogenic/brown fat-like genes by qPCR. (C) Primary inguinal SV cells were purified as in (A) and differentiated into adipocytes for 6 days in the presence of FC fragment of human IgG (hFC) (100nM), fusion protein of irisin-FC (100nM) or recombinant FNDC5 (20nM). Total RNA was isolated and assayed for mRNA levels of general adipogenic marker aP2, thermogenic gene Ucp-1 and other brown-like genes by qPCR. Values are mean±SD (n=3). *, p<0.05, **, p<0.01, ***, p<0.001. Similar results were observed in more than three independent experiments.
Figure 5. “Brown” fat in human adults share molecular characteristics of murine beige rather than brown cells
Expression of beige- and brown-selective genes in hWAT versus hBAT samples from total 11 subjects of two independent cohorts were measured by qPCR. (A) UCP1 expression levels were measured and confirmed to be higher in BAT as compared to WAT. (B) Heatmap summary of relative fold changes in expression of these genes in each subject. Relative gene expression level of 3 beige-selective (C), 3 brown-selective (D) are shown individually. P value was analyzed using Wilcoxom matched pairs signed ranks test.
Figure 6. Beige selective markers are expressed in human “brown” fat cells residing in supraclavicular regions
Microscopic images of adult supraclavicular adipose tissue stained with antibodies recognizing CD137 (red, A and C), UCP1 (green, A), TMEM26 (green, B) and perilipin (green, C) as indicated with nuclei stained with DAPI. It is worth noting that in (A) and (B) the expression of both CD137 and TMEM26 in multilocular (see the arrowhead in B), UCP1+ adipocytes (labeled with “BAT”), but the neighboring perilipin-1+, UCP1− adipocytes are CD137− (labeled with “WAT”, see C).
Similar articles
- Thermogenic ability of uncoupling protein 1 in beige adipocytes in mice.
Okamatsu-Ogura Y, Fukano K, Tsubota A, Uozumi A, Terao A, Kimura K, Saito M. Okamatsu-Ogura Y, et al. PLoS One. 2013 Dec 30;8(12):e84229. doi: 10.1371/journal.pone.0084229. eCollection 2013. PLoS One. 2013. PMID: 24386355 Free PMC article. - Thermogenic activity of UCP1 in human white fat-derived beige adipocytes.
Bartesaghi S, Hallen S, Huang L, Svensson PA, Momo RA, Wallin S, Carlsson EK, Forslöw A, Seale P, Peng XR. Bartesaghi S, et al. Mol Endocrinol. 2015 Jan;29(1):130-9. doi: 10.1210/me.2014-1295. Mol Endocrinol. 2015. PMID: 25389910 Free PMC article. - Induction of beige-like adipocytes in 3T3-L1 cells.
Asano H, Kanamori Y, Higurashi S, Nara T, Kato K, Matsui T, Funaba M. Asano H, et al. J Vet Med Sci. 2014 Jan;76(1):57-64. doi: 10.1292/jvms.13-0359. Epub 2013 Sep 20. J Vet Med Sci. 2014. PMID: 24065084 Free PMC article. - Is the heat surrounding adipose tissue mitochondria warranted?
Porter C, Malagaris I, Sidossis LS. Porter C, et al. Curr Opin Clin Nutr Metab Care. 2014 Nov;17(6):503-8. doi: 10.1097/MCO.0000000000000102. Curr Opin Clin Nutr Metab Care. 2014. PMID: 25102333 Free PMC article. Review. - Brown and Beige Fat: Physiological Roles beyond Heat Generation.
Kajimura S, Spiegelman BM, Seale P. Kajimura S, et al. Cell Metab. 2015 Oct 6;22(4):546-59. doi: 10.1016/j.cmet.2015.09.007. Cell Metab. 2015. PMID: 26445512 Free PMC article. Review.
Cited by
- Ubiquitination and Metabolic Disease.
Ma M, Cao R, Tian Y, Fu X. Ma M, et al. Adv Exp Med Biol. 2024;1466:47-79. doi: 10.1007/978-981-97-7288-9_4. Adv Exp Med Biol. 2024. PMID: 39546135 Review. - Two Regions with Different Expression of Lipogenic Enzymes in Rats' Posterior Subcutaneous Fat Depot.
Turyn J, Stelmanska E, Szrok-Jurga S. Turyn J, et al. Int J Mol Sci. 2024 Oct 27;25(21):11546. doi: 10.3390/ijms252111546. Int J Mol Sci. 2024. PMID: 39519099 Free PMC article. - Depot-specific mRNA expression programs in human adipocytes suggest physiological specialization via distinct developmental programs.
Clemons HJ, Hogan DJ, Brown PO. Clemons HJ, et al. PLoS One. 2024 Oct 14;19(10):e0311751. doi: 10.1371/journal.pone.0311751. eCollection 2024. PLoS One. 2024. PMID: 39401200 Free PMC article. - Keys to the switch of fat burning: stimuli that trigger the uncoupling protein 1 (UCP1) activation in adipose tissue.
Gong D, Lei J, He X, Hao J, Zhang F, Huang X, Gu W, Yang X, Yu J. Gong D, et al. Lipids Health Dis. 2024 Sep 28;23(1):322. doi: 10.1186/s12944-024-02300-z. Lipids Health Dis. 2024. PMID: 39342273 Free PMC article. Review. - Cold Exposure Rejuvenates the Metabolic Phenotype of Panx1-/- Mice.
Molica F, Ehrlich A, Pelli G, Rusiecka OM, Montessuit C, Chanson M, Kwak BR. Molica F, et al. Biomolecules. 2024 Aug 25;14(9):1058. doi: 10.3390/biom14091058. Biomolecules. 2024. PMID: 39334824 Free PMC article.
References
- Billon N, Dani C. Developmental Origins of the Adipocyte Lineage: New Insights from Genetics and Genomics Studies. Stem Cell Rev 2011 - PubMed
- Cinti S. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest. 2002;25:823–835. - PubMed
- Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol. 2004;18:2123–2131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK90861/DK/NIDDK NIH HHS/United States
- RC4 DK090861/DK/NIDDK NIH HHS/United States
- K01 DK094824/DK/NIDDK NIH HHS/United States
- DK31405/DK/NIDDK NIH HHS/United States
- R37 DK031405/DK/NIDDK NIH HHS/United States
- R01 DK031405/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous