Nutritional immunity: transition metals at the pathogen-host interface - PubMed (original) (raw)
Review
Nutritional immunity: transition metals at the pathogen-host interface
M Indriati Hood et al. Nat Rev Microbiol. 2012.
Abstract
Transition metals occupy an essential niche in biological systems. Their electrostatic properties stabilize substrates or reaction intermediates in the active sites of enzymes, and their heightened reactivity is harnessed for catalysis. However, this heightened activity also renders transition metals toxic at high concentrations. Bacteria, like all living organisms, must regulate their intracellular levels of these elements to satisfy their physiological needs while avoiding harm. It is therefore not surprising that the host capitalizes on both the essentiality and toxicity of transition metals to defend against bacterial invaders. This Review discusses established and emerging paradigms in nutrient metal homeostasis at the pathogen-host interface.
Figures
Figure 1. Fe limitation and Fe acquisition during bacterial infections
a. Overview of Fe limitation strategies in the vertebrate host. Fe3+ is stored intracellularly in complex with ferritin (F), bound by serum transferrin (TF) or bound by lactoferrin (LTF) at mucosal surfaces. In the blood, Fe2+ is complexed with haem, which is bound by haemoglobin within red blood cells (RBCs). Upon red cell lysis, haemoglobin is bound by haptoglobin (Hpt) and free haem is scavenged by haemopexin (Hpx). In addition to these haem-binding proteins, neutrophil gelatinase-associated lipocalin (NGAL) binds and sequesters bacterial siderophores. b. Representative Fe acquisition systems expressed by Gram-negative and Gram-positive pathogens. Both Gram-negative and Gram-positive pathogens possess systems to acquire Fe3+-siderophores, Fe2+-haem, Fe3+ from transferrin and/or free Fe2+. Not all systems are expressed by the same organism. TF, transferrin; SIP, siderophore interacting protein; HO, haem oxygenase; Hpt, haptoglobin; OM, outer membrane; P, periplasm; IM, inner membrane; CW, cell wall; CM, cytoplasmic membrane.
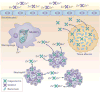
Figure 2. Mn and Zn homeostasis at the pathogen-host interface
a. Zn2+ and Mn2+ sequestration by S100 family proteins at epithelial surfaces and within tissue abscesses. S100A7 is released at epithelial surfaces, where it inhibits bacterial invasion through chelation of Zn2+. In deep tissues, infection leads to recruitment of neutrophils, which deliver calprotectin (S100A8/A9) to the infection site. Calprotectin inhibits bacterial growth through chelation of Mn2+ and Zn2+ and is thought to be transported away from the abscess by an as-yet-unknown mechanism. Engulfment of bacteria by macrophages leads to decreased Zn2+ uptake and increased Zn2+ efflux from the cytoplasm and efflux of Mn2+ and Fe from the phagosome by NRAMP1. b. Representative Mn2+ and Zn2+ uptake systems expressed by pathogenic bacteria. c. Proposed mechanisms of Zn2+ intoxication employed by the host and Zn2+ detoxification systems expressed by pathogens. Upon infection, Zn accumulates in the phagolysosome where it is toxic to bacteria. Gram-negative and Gram-positive bacteria primarily alleviate Zn toxicity through efflux of excess Zn from the cytoplasm. d. Proposed mechanism for Zn toxicity in bacteria. When the extracellular Zn2+: Mn2+ ratio is high, Zn2+ binds the SBP of Mn2+-specific transporters, preventing Mn2+ binding and uptake.
Figure 3. New insights into the roles for Cu in innate immunity
a. Mechanisms of Cu intoxication within macrophages. Following phagocytosis of bacteria, interferon gamma induces expression of the Cu+ importer Ctr1. Cu is bound by Atox1 and shuttled to the phagosomal Cu+ transporter, ATP7A. Accumulation of copper within the phagolysosome contributes to bacterial killing through multiple mechanisms including disruption of Fe-S cluster-containing bacterial proteins and possibly through the generation of reactive oxygen species. This leads to inhibition of bacterial metabolic processes and damage to DNA, proteins and lipids. b. Cu detoxification systems expressed by pathogenic bacteria. Bacteria encode multiple mechanisms to detoxify the cytoplasm or periplasm from excess Cu+ including expression of Cu+ efflux systems, periplasmic multicopper oxidases and cytoplasmic Cu chaperones. Many bacteria express several independently regulated Cu detoxification systems, which provide a graded response to Cu toxicity.
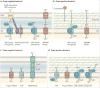
Figure 4
a | Mn2+ and Zn2+ uptake systems in pathogenic Gram-negative bacteria. b | Mn2+ and Zn2+ uptake systems in pathogenic Gram-positive bacteria. c | Proposed mechanisms of Zn2+ detoxification by Gram-negative pathogens. On infection, Zn2+ accumulates in the phagolysosome, where it is toxic to bacteria. Gram-negative bacteria alleviate this Zn2+ toxicity primarily through efflux of excess Zn2+ from the cytoplasm. d | Proposed mechanisms of Zn2+ and Mn2+ detoxification by Gram-positive pathogens. Zn2+ can be exported from the bacterial cytoplasm by dedicated transporters. Mn2+ is usually imported by the ABC family pneumococcal surface adhesin (Psa) system, but when the extracellular Zn2+/Mn2+ ratio is high, Zn2+ binds PsaA, the substrate-binding protein of this Mn2+-specific transporter, preventing Mn2+ binding and uptake. CDF, cation diffusion facilitator; CM, cytoplasmic membrane; IM, inner membrane; MntH, Mn transport H; OM, outer membrane; Znu, Zn uptake protein.
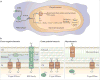
Figure 5
a | Mechanisms of Cu+ intoxication within macrophages. Following phagocytosis of bacteria, interferon-γ induces expression of the Cu+ importer Cu+ transport protein 1 (CTR1). Cu+ is bound by ATOX1 and shuttled to the phagosomal Cu+ transporter, ATP7A. Accumulation of Cu+ within the phagolysosome contributes to bacterial killing through multiple mechanisms, including the disruption of Fe–S cluster-containing bacterial proteins, and possibly the generation of reactive oxygen species. This leads to inhibition of bacterial metabolic processes and damage to DNA, proteins and lipids. b | Pathogenic bacteria encode multiple systems to detoxify the cytoplasm or periplasm when there is excess Cu+, including Cu+ efflux systems (such as the RND family transporter CusABC), periplasmic multicopper oxidases (such as blue Cu oxidase (CueO)) and cytoplasmic Cu+ chaperones (such as metallothionein (MymT)). Many bacteria express several independently regulated Cu+ detoxification systems, providing a graded response to Cu+ toxicity. CM, cytoplasmic membrane; IM, inner membrane; OM, outer membrane; TCA, tricarboxylic acid.
Similar articles
- Metallobiology of Tuberculosis.
Marcela Rodriguez G, Neyrolles O. Marcela Rodriguez G, et al. Microbiol Spectr. 2014 Jun;2(3):10.1128/microbiolspec.MGM2-0012-2013. doi: 10.1128/microbiolspec.MGM2-0012-2013. Microbiol Spectr. 2014. PMID: 26103977 Free PMC article. Review. - Metal limitation and toxicity at the interface between host and pathogen.
Becker KW, Skaar EP. Becker KW, et al. FEMS Microbiol Rev. 2014 Nov;38(6):1235-49. doi: 10.1111/1574-6976.12087. Epub 2014 Sep 29. FEMS Microbiol Rev. 2014. PMID: 25211180 Free PMC article. Review. - Multi-metal nutrient restriction and crosstalk in metallostasis systems in microbial pathogens.
Jordan MR, Wang J, Capdevila DA, Giedroc DP. Jordan MR, et al. Curr Opin Microbiol. 2020 Jun;55:17-25. doi: 10.1016/j.mib.2020.01.010. Epub 2020 Feb 12. Curr Opin Microbiol. 2020. PMID: 32062305 Free PMC article. Review. - Transition Metals and Virulence in Bacteria.
Palmer LD, Skaar EP. Palmer LD, et al. Annu Rev Genet. 2016 Nov 23;50:67-91. doi: 10.1146/annurev-genet-120215-035146. Epub 2016 Sep 7. Annu Rev Genet. 2016. PMID: 27617971 Free PMC article. Review. - The roles of transition metals in the physiology and pathogenesis of Streptococcus pneumoniae.
Honsa ES, Johnson MD, Rosch JW. Honsa ES, et al. Front Cell Infect Microbiol. 2013 Dec 4;3:92. doi: 10.3389/fcimb.2013.00092. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24364001 Free PMC article. Review.
Cited by
- A chemical screen identifies structurally diverse metal chelators with activity against the fungal pathogen Candida albicans.
Fallah S, Duncan D, Reichl KD, Smith MJ, Wang W, Porco JA Jr, Brown LE, Whitesell L, Robbins N, Cowen LE. Fallah S, et al. Microbiol Spectr. 2024 Apr 2;12(4):e0409523. doi: 10.1128/spectrum.04095-23. Epub 2024 Feb 20. Microbiol Spectr. 2024. PMID: 38376363 Free PMC article. - Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper.
Stafford SL, Bokil NJ, Achard ME, Kapetanovic R, Schembri MA, McEwan AG, Sweet MJ. Stafford SL, et al. Biosci Rep. 2013 Jul 16;33(4):e00049. doi: 10.1042/BSR20130014. Biosci Rep. 2013. PMID: 23738776 Free PMC article. Review. - Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems.
Huang X, Shin JH, Pinochet-Barros A, Su TT, Helmann JD. Huang X, et al. Mol Microbiol. 2017 Jan;103(2):253-268. doi: 10.1111/mmi.13554. Epub 2016 Nov 2. Mol Microbiol. 2017. PMID: 27748968 Free PMC article. - Increasing the copper sensitivity of microorganisms by restricting iron supply, a strategy for bio-management practices.
Steunou AS, Bourbon ML, Babot M, Durand A, Liotenberg S, Yamaichi Y, Ouchane S. Steunou AS, et al. Microb Biotechnol. 2020 Sep;13(5):1530-1545. doi: 10.1111/1751-7915.13590. Epub 2020 Jun 19. Microb Biotechnol. 2020. PMID: 32558275 Free PMC article. - Methionine-rich domains emerge as facilitators of copper recruitment in detoxification systems.
Contaldo U, Savant-Aira D, Vergnes A, Becam J, Biaso F, Ilbert M, Aussel L, Ezraty B, Lojou E, Mazurenko I. Contaldo U, et al. Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2402862121. doi: 10.1073/pnas.2402862121. Epub 2024 Oct 8. Proc Natl Acad Sci U S A. 2024. PMID: 39378088 Free PMC article.
References
- Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. Journal of Biological Inorganic Chemistry. 2008;13:1205–1218. - PubMed
- Andreini C, Bertini I, Rosato A. Metalloproteomes: a bioinformatic approach. Accounts of chemical research. 2009;42:1471–1479. - PubMed
- Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. Journal of Proteome Research. 2006;5:3173–3178. - PubMed
- Weinberg ED. Nutritional immunity. Host's attempt to withold iron from microbial invaders. Journal ofthe American Medical Association. 1975;231:39–41. - PubMed
- Weinberg ED. Iron availability and infection. Biochimica et Biophysica Acta. 2009;1790:600–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI069233/AI/NIAID NIH HHS/United States
- AI069233/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- AI073843/AI/NIAID NIH HHS/United States
- AI091771/AI/NIAID NIH HHS/United States
- R01 AI073843/AI/NIAID NIH HHS/United States
- R56 AI091771/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources