The mechanism of patellamide macrocyclization revealed by the characterization of the PatG macrocyclase domain - PubMed (original) (raw)
doi: 10.1038/nsmb.2340. Epub 2012 Jul 15.
Andrew Bent, Wael E Houssen, David Zollman, Falk Morawitz, Sally Shirran, Jeremie Vendome, Ada F Nneoyiegbe, Laurent Trembleau, Catherine H Botting, Margaret C M Smith, Marcel Jaspars, James H Naismith
Affiliations
- PMID: 22796963
- PMCID: PMC3462482
- DOI: 10.1038/nsmb.2340
The mechanism of patellamide macrocyclization revealed by the characterization of the PatG macrocyclase domain
Jesko Koehnke et al. Nat Struct Mol Biol. 2012 Aug.
Abstract
Peptide macrocycles are found in many biologically active natural products. Their versatility, resistance to proteolysis and ability to traverse membranes has made them desirable molecules. Although technologies exist to synthesize such compounds, the full extent of diversity found among natural macrocycles has yet to be achieved synthetically. Cyanobactins are ribosomal peptide macrocycles encompassing an extraordinarily diverse range of ring sizes, amino acids and chemical modifications. We report the structure, biochemical characterization and initial engineering of the PatG macrocyclase domain of Prochloron sp. from the patellamide pathway that catalyzes the macrocyclization of linear peptides. The enzyme contains insertions in the subtilisin fold to allow it to recognize a three-residue signature, bind substrate in a preorganized and unusual conformation, shield an acyl-enzyme intermediate from water and catalyze peptide bond formation. The ability to macrocyclize a broad range of nonactivated substrates has wide biotechnology applications.
Figures
Figure 1
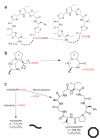
Macrocyclization of patellamides. (a) The PatE prepropeptide consists of an N-terminal leader sequence followed by two eight-residue cassettes with the C-terminal macrocyclase recognition signal AYDG. X indicates any amino acid. The macrocyclization domain of PatG catalyzes the formation (dashed lines) of two cyclic peptides per prepropeptide. (b) PatGmac requires a heterocycle or proline (denoted Z) at the P1 position and the AYDG motif at the P1′–P4′ sites. An additional glutamate is often found at P5′ but is not required. (c) The test substrate used in this study can either give a linear peptide (curved line) with a mass of 716.375 Da or a macrocycle (octagon), which is 18 Da lighter.
Figure 2
Structure of PatGmac. (a) PatGmac has a subtilisin-like core (cyan) and the conventional catalytic triad (yellow sticks). PatGmac has an insertion (magenta) that extends from β2 as a loop, then forms a helix-loop-helix motif and creates an N-terminal extension of α4, the helix that harbors His618. The insertion is found in other macrocyclases but is not conserved in length or sequence. (b) Difference electron density (_F_o – _F_c contoured at 3σ with phases calculated from a model which was refined with no peptide present) of the PIPFPAYDG substrate mimic bound to PatGmac H618A; three N-terminal residues (VPA) of the substrate mimic are disordered. (c) Interactions between the substrate mimic and PatGmac H618A. The proline (at position P1) of the substrate adopts a cis peptide conformation that results in the substrate pointing away from the protein. The side chains of Met660, Phe684 and Arg686 would prevent the binding of substrates that adopt an extended conformation. Lys598 and possibly Lys594 form salt bridges (dashed lines) with the P3′ residue (aspartate), whereas the P2′ tyrosine forms a hydrogen bond (dashed line) with His746 and interacts with Phe747 through π-stacking. (d) The active site where the acyl-enzyme intermediate would be formed is shielded from solvent by the macrocyclization insertion and the AYDG peptide (dark green).
Figure 3
Biochemical characterization of PatGmac and PatGmac mutants. (a–c) LC-ESI MS of macrocyclization reactions with PatGmac wild type (a), PatGmacΔ2 (b) and PatGmac K594D (c). Macrocyclized and linear products are indicated with octagons and curved lines, respectively. The error between observed and calculated mass is shown below the [M+H]+ and [M+Na]+ species. (d) Evidence for a stable acyl-enzyme intermediate between PatGmac and substrate.
Figure 4
Proposed mechanism for macrocyclization. (a) Model of the acyl-enzyme intermediate with AYDG remaining bound at the active site. (b) The acyl-enzyme intermediate is in equilibrium with the substrate. In PatGmac the N terminus of the substrate enters the active site, displacing AYDG and leading to macrocyclization. Mutations that disrupt binding of AYDG lead to linear product, as the substrate is hydrolyzed by water. The role of the histidine in deprotonating the incoming N terminus is speculative.
Similar articles
- Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella.
Schmidt EW, Nelson JT, Rasko DA, Sudek S, Eisen JA, Haygood MG, Ravel J. Schmidt EW, et al. Proc Natl Acad Sci U S A. 2005 May 17;102(20):7315-20. doi: 10.1073/pnas.0501424102. Epub 2005 May 9. Proc Natl Acad Sci U S A. 2005. PMID: 15883371 Free PMC article. - Possible Functional Roles of Patellamides in the Ascidian-Prochloron Symbiosis.
Baur P, Kühl M, Comba P, Behrendt L. Baur P, et al. Mar Drugs. 2022 Feb 2;20(2):119. doi: 10.3390/md20020119. Mar Drugs. 2022. PMID: 35200648 Free PMC article. Review. - Structure of PatF from Prochloron didemni.
Bent AF, Koehnke J, Houssen WE, Smith MC, Jaspars M, Naismith JH. Bent AF, et al. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013 Jun;69(Pt 6):618-23. doi: 10.1107/S1744309113012931. Epub 2013 May 23. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013. PMID: 23722837 Free PMC article. - Insights into the Mechanism of the Cyanobactin Heterocyclase Enzyme.
Ge Y, Czekster CM, Miller OK, Botting CH, Schwarz-Linek U, Naismith JH. Ge Y, et al. Biochemistry. 2019 Apr 23;58(16):2125-2132. doi: 10.1021/acs.biochem.9b00084. Epub 2019 Apr 5. Biochemistry. 2019. PMID: 30912640 Free PMC article. - Copper coordination chemistry of the patellamides - cyanobactins in the ascidian-Prochloron symbiosis.
Baur P, Comba P. Baur P, et al. Dalton Trans. 2025 Mar 4;54(10):3968-3976. doi: 10.1039/d4dt03002h. Dalton Trans. 2025. PMID: 39670798 Review.
Cited by
- Chemoenzymatic Late-Stage Modifications Enable Downstream Click-Mediated Fluorescent Tagging of Peptides.
Colombano A, Dalponte L, Dall'Angelo S, Clemente C, Idress M, Ghazal A, Houssen WE. Colombano A, et al. Angew Chem Int Ed Engl. 2023 Apr 11;62(16):e202215979. doi: 10.1002/anie.202215979. Epub 2023 Mar 10. Angew Chem Int Ed Engl. 2023. PMID: 36815722 Free PMC article. - Assessing the combinatorial potential of the RiPP cyanobactin tru pathway.
Ruffner DE, Schmidt EW, Heemstra JR. Ruffner DE, et al. ACS Synth Biol. 2015 Apr 17;4(4):482-92. doi: 10.1021/sb500267d. Epub 2014 Sep 2. ACS Synth Biol. 2015. PMID: 25140729 Free PMC article. - Efficient backbone cyclization of linear peptides by a recombinant asparaginyl endopeptidase.
Harris KS, Durek T, Kaas Q, Poth AG, Gilding EK, Conlan BF, Saska I, Daly NL, van der Weerden NL, Craik DJ, Anderson MA. Harris KS, et al. Nat Commun. 2015 Dec 18;6:10199. doi: 10.1038/ncomms10199. Nat Commun. 2015. PMID: 26680698 Free PMC article. - New Insights into the Biosynthetic Logic of Ribosomally Synthesized and Post-translationally Modified Peptide Natural Products.
Ortega MA, van der Donk WA. Ortega MA, et al. Cell Chem Biol. 2016 Jan 21;23(1):31-44. doi: 10.1016/j.chembiol.2015.11.012. Cell Chem Biol. 2016. PMID: 26933734 Free PMC article. Review. - Expanding the chemical space of synthetic cyclic peptides using a promiscuous macrocyclase from prenylagaramide biosynthesis.
Sarkar S, Gu W, Schmidt EW. Sarkar S, et al. ACS Catal. 2020 Jul 2;10(13):7146-7153. doi: 10.1021/acscatal.0c00623. Epub 2020 Jun 17. ACS Catal. 2020. PMID: 33457065 Free PMC article.
References
- Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Nat. Prod. Rep. 2012;29:144–222. - PubMed
- Mayer AM, Rodriguez AD, Berlinck RG, Fusetani N. Marine pharmacology in 2007–8: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011;153:191–222. - PMC - PubMed
- Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery–an underexploited structural class. Nat. Rev. Drug Discov. 2008;7:608–624. - PubMed
- Cuevas C, Francesch A. Development of Yondelis (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat. Prod. Rep. 2009;26:322–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 086036/WT_/Wellcome Trust/United Kingdom
- BB/H001212/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H005447/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F003439/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials