Position-dependent FUS-RNA interactions regulate alternative splicing events and transcriptions - PubMed (original) (raw)
Position-dependent FUS-RNA interactions regulate alternative splicing events and transcriptions
Shinsuke Ishigaki et al. Sci Rep. 2012.
Erratum in
- Sci Rep. 2013;3:3301
Abstract
FUS is an RNA-binding protein that regulates transcription, alternative splicing, and mRNA transport. Aberrations of FUS are causally associated with familial and sporadic ALS/FTLD. We analyzed FUS-mediated transcriptions and alternative splicing events in mouse primary cortical neurons using exon arrays. We also characterized FUS-binding RNA sites in the mouse cerebrum with HITS-CLIP. We found that FUS-binding sites tend to form stable secondary structures. Analysis of position-dependence of FUS-binding sites disclosed scattered binding of FUS to and around the alternatively spliced exons including those associated with neurodegeneration such as Mapt, Camk2a, and Fmr1. We also found that FUS is often bound to the antisense RNA strand at the promoter regions. Global analysis of these FUS-tags and the expression profiles disclosed that binding of FUS to the promoter antisense strand downregulates transcriptions of the coding strand. Our analysis revealed that FUS regulates alternative splicing events and transcriptions in a position-dependent manner.
Figures
Figure 1. Experimental schemes.
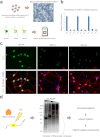
(a) Mouse primary cortical neurons are prepared and infected with lentivirus expressing two different shRNA against FUS (shFus1 and shFus2) and control shRNA (shCont). Total RNA is isolated and analyzed by the Affymetrix Mouse Exon Array. (b) Fus is efficiently knocked down in primary cortical neurons, which is evaluated by real-time qRT-PCR. Bars indicate the mean and SD of three experiments. (c) Immunohistchimical analysis using anti-FUS antibody on primary cortical neurons silenced by shFus1, shFus2, and shCont. Cells are fixed and immunostained with anti-FUS antibody, anti-βTubulin antibody, and DAPI. (d) Mouse cerebrum derived from a 12-week-old C57Bl/6 mouse is UV-irradiated at 400 mJ and FUS-bound RNA segments are immuno-precipitated. High-throughput 50 bp single-end sequencing is performed using the SOLiD 3 sequencer.
Figure 2. Four representative FUS-mediated alternative splicing events.
The top panels show the positions of CLIP-tags and exon-intron structures. The second panels represent schematic splicing changes mediated by FUS. shCont and shFus lead to the upper and lower splicing events, respectively. The third panels show representative RT-PCR of the indicated exons. The experiments are repeated in quadruplicate using four independent sets of samples. The last panels show densitometric quantification of RT-PCR (n = 4; mean and SD).
Figure 3. Annotation mapping of FUS CLIP-tags.
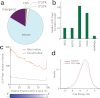
(a) Distributions of FUS CLIP-tags. Binding regions are mapped to CDS (coding sequence), 5′ and 3′ UTRs, introns, intergenic regions including tRNA and rRNA genes according to the ENSEMBL version e!61 annotation based on the mouse genome assembly NCBI build 37.1/mm9. Pie-charts show ratios of binding regions mapped to the indicated regions. (b) Distribution of FUS CLIP-tags normalized for the length of each annotation. (c) Distribution of FUS CLIP-tags mapped to the relative positions of each gene. The broken line indicates 12,508 genes with constitutive transcriptional start/end sites, and the solid line indicates 7,477 genes with alternative transcriptional start/end sites. (d) Probability density function of the minimum free energies, δG, of 30-mer stretches of CLIP-tagged regions. CLIP-tagged regions and controls are shown in red and blue lines, respectively.
Figure 4. Mapping of CLIP-tags on exon-intron structures.
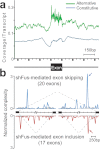
(a) Distribution of CLIP-tags on constitutively or alternatively spliced exons and the flanking intronic regions. The abscissa indicates an intron-exon-intron structure. The sizes of all the exons are normalized to 150 nucleotides. The number of exonic CLIP-tags is also normalized accordingly. Intronic CLIP-tags within 1,000 nucleotides upstream or downstream of exons are indicated. The number of CLIP-tags is normalized for the number of transcripts belonging to each category of constitutive and alternative exons. (b) Normalized complexity map of FUS-dependent splice sites. shFus-mediated alternative splicing events are compiled. Arrows point to conspicuous peaks at ∼500 nt upstream of the 3′ end of the downstream intron. Shaded areas indicate an average of 100 sets of normalized complexity of 20 randomly selected constitutive exons.
Figure 5. CLIP-tags on the promoter antisense strand and gene expression profiles.
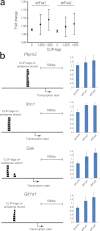
(a) The numbers of CLIP-tags on the antisense strand at 1 to 700 nucleotides upstream of the transcription start sites of each gene are divided into three categories according to the partitioning functionality of the JMP 8.0 software. Fold-changes of gene expression levels with shFus1 and shFus2 are calculated for each category. Means and SEs are plotted. The numbers of transcription start sites are 2390 for no tag, 53 for 1-250 tags, and 18 for more than 250 tags. The number of CLIP-tags represents the total number of nucleotides covered by the tags. No statistical difference is observed for each dataset with the one-way ANOVA analysis. (b) Four representative genes, Ptprn2, Xrn1, Gak, and Glt1d1, for which more than 250 CLIP-tags are bound to the promoter antisense strand, are validated by real-time qPCR. Changes in gene expression levels in cortical neurons after knocking down Fus with shFus1 and shFus2 are indicated by means and SDs (n = 3).
Similar articles
- FUS-mediated regulation of alternative RNA processing in neurons: insights from global transcriptome analysis.
Masuda A, Takeda J, Ohno K. Masuda A, et al. Wiley Interdiscip Rev RNA. 2016 May;7(3):330-40. doi: 10.1002/wrna.1338. Epub 2016 Jan 28. Wiley Interdiscip Rev RNA. 2016. PMID: 26822113 Review. - Distinct and shared functions of ALS-associated proteins TDP-43, FUS and TAF15 revealed by multisystem analyses.
Kapeli K, Pratt GA, Vu AQ, Hutt KR, Martinez FJ, Sundararaman B, Batra R, Freese P, Lambert NJ, Huelga SC, Chun SJ, Liang TY, Chang J, Donohue JP, Shiue L, Zhang J, Zhu H, Cambi F, Kasarskis E, Hoon S, Ares M Jr, Burge CB, Ravits J, Rigo F, Yeo GW. Kapeli K, et al. Nat Commun. 2016 Jul 5;7:12143. doi: 10.1038/ncomms12143. Nat Commun. 2016. PMID: 27378374 Free PMC article. - FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons.
Errichelli L, Dini Modigliani S, Laneve P, Colantoni A, Legnini I, Capauto D, Rosa A, De Santis R, Scarfò R, Peruzzi G, Lu L, Caffarelli E, Shneider NA, Morlando M, Bozzoni I. Errichelli L, et al. Nat Commun. 2017 Mar 30;8:14741. doi: 10.1038/ncomms14741. Nat Commun. 2017. PMID: 28358055 Free PMC article. - FUS interacts with nuclear matrix-associated protein SAFB1 as well as Matrin3 to regulate splicing and ligand-mediated transcription.
Yamaguchi A, Takanashi K. Yamaguchi A, et al. Sci Rep. 2016 Oct 12;6:35195. doi: 10.1038/srep35195. Sci Rep. 2016. PMID: 27731383 Free PMC article. - [The FUS protein: Physiological functions and a role in amyotrophic lateral sclerosis].
Efimova AD, Ovchinnikov RK, Roman AY, Maltsev AV, Grigoriev VV, Kovrazhkina EA, Skvortsova VI. Efimova AD, et al. Mol Biol (Mosk). 2017 May-Jun;51(3):387-399. doi: 10.7868/S0026898417020094. Mol Biol (Mosk). 2017. PMID: 28707655 Review. Russian.
Cited by
- Nuclear Envelope and Nuclear Pore Complexes in Neurodegenerative Diseases-New Perspectives for Therapeutic Interventions.
Hachiya N, Sochocka M, Brzecka A, Shimizu T, Gąsiorowski K, Szczechowiak K, Leszek J. Hachiya N, et al. Mol Neurobiol. 2021 Mar;58(3):983-995. doi: 10.1007/s12035-020-02168-x. Epub 2020 Oct 17. Mol Neurobiol. 2021. PMID: 33067781 Free PMC article. Review. - CLIPing the brain: studies of protein-RNA interactions important for neurodegenerative disorders.
Modic M, Ule J, Sibley CR. Modic M, et al. Mol Cell Neurosci. 2013 Sep;56:429-35. doi: 10.1016/j.mcn.2013.04.002. Epub 2013 Apr 10. Mol Cell Neurosci. 2013. PMID: 23583633 Free PMC article. Review. - Mutant FUS and ELAVL4 (HuD) Aberrant Crosstalk in Amyotrophic Lateral Sclerosis.
De Santis R, Alfano V, de Turris V, Colantoni A, Santini L, Garone MG, Antonacci G, Peruzzi G, Sudria-Lopez E, Wyler E, Anink JJ, Aronica E, Landthaler M, Pasterkamp RJ, Bozzoni I, Rosa A. De Santis R, et al. Cell Rep. 2019 Jun 25;27(13):3818-3831.e5. doi: 10.1016/j.celrep.2019.05.085. Cell Rep. 2019. PMID: 31242416 Free PMC article. - Bioinformatic analysis and prediction of the function and regulatory network of long non-coding RNAs in hepatocellular carcinoma.
Cao MR, Han ZP, Liu JM, Li YG, Lv YB, Zhou JB, He JH. Cao MR, et al. Oncol Lett. 2018 May;15(5):7783-7793. doi: 10.3892/ol.2018.8271. Epub 2018 Mar 15. Oncol Lett. 2018. PMID: 29740493 Free PMC article. - tRIP-seq reveals repression of premature polyadenylation by co-transcriptional FUS-U1 snRNP assembly.
Masuda A, Kawachi T, Takeda JI, Ohkawara B, Ito M, Ohno K. Masuda A, et al. EMBO Rep. 2020 May 6;21(5):e49890. doi: 10.15252/embr.201949890. Epub 2020 Mar 18. EMBO Rep. 2020. PMID: 32189459 Free PMC article.
References
- Strong M. J. & Volkening K. TDP-43 and FUS/TLS: sending a complex message about messenger RNA in amyotrophic lateral sclerosis? FEBS J 278, 3569–3577 (2011). - PubMed
- Kwiatkowski T. J. Jr et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous