ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation - PubMed (original) (raw)
. 2012 Jul 25;487(7408):477-81.
doi: 10.1038/nature11228.
Thomas Perlot, Ateequr Rehman, Jean Trichereau, Hiroaki Ishiguro, Magdalena Paolino, Verena Sigl, Toshikatsu Hanada, Reiko Hanada, Simone Lipinski, Birgit Wild, Simone M R Camargo, Dustin Singer, Andreas Richter, Keiji Kuba, Akiyoshi Fukamizu, Stefan Schreiber, Hans Clevers, Francois Verrey, Philip Rosenstiel, Josef M Penninger
Affiliations
- PMID: 22837003
- PMCID: PMC7095315
- DOI: 10.1038/nature11228
ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation
Tatsuo Hashimoto et al. Nature. 2012.
Abstract
Malnutrition affects up to one billion people in the world and is a major cause of mortality. In many cases, malnutrition is associated with diarrhoea and intestinal inflammation, further contributing to morbidity and death. The mechanisms by which unbalanced dietary nutrients affect intestinal homeostasis are largely unknown. Here we report that deficiency in murine angiotensin I converting enzyme (peptidyl-dipeptidase A) 2 (Ace2), which encodes a key regulatory enzyme of the renin-angiotensin system (RAS), results in highly increased susceptibility to intestinal inflammation induced by epithelial damage. The RAS is known to be involved in acute lung failure, cardiovascular functions and SARS infections. Mechanistically, ACE2 has a RAS-independent function, regulating intestinal amino acid homeostasis, expression of antimicrobial peptides, and the ecology of the gut microbiome. Transplantation of the altered microbiota from Ace2 mutant mice into germ-free wild-type hosts was able to transmit the increased propensity to develop severe colitis. ACE2-dependent changes in epithelial immunity and the gut microbiota can be directly regulated by the dietary amino acid tryptophan. Our results identify ACE2 as a key regulator of dietary amino acid homeostasis, innate immunity, gut microbial ecology, and transmissible susceptibility to colitis. These results provide a molecular explanation for how amino acid malnutrition can cause intestinal inflammation and diarrhoea.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
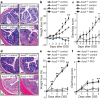
Figure 1. Ace2 deficiency and protein malnutrition worsen DSS-induced colitis.
a, Colon histopathology, b, percentage weight loss, and c, diarrhoea scores in control and DSS-treated Ace2 +/y and Ace2 −/y littermates. In a, note crypt damage (arrowheads), ulcerations (arrow), and infiltration of inflammatory cells (asterisks) in DSS-treated Ace2 −/y mice. Haematoxylin and eosin staining on day 7 after DSS challenge. Scale bars, 100 μm. d, Colon histopathology (haematoxylin and eosin staining, day 4 after DSS challenge; scale bars, 100 μm), e, percentage weight loss, and f, diarrhoea scores of DSS-treated Ace2 +/y and Ace2 −/y littermates fed either normal chow (Control) or a protein free diet (PFD; <0.2% protein). All values are mean ± s.e.m. of 5–9 mice per group. *P < 0.05, **P < 0.01 comparing DSS-treated Ace2 +/y with Ace2 −/y littermates, or Ace2 +/y mice on normal diet with those on PFD (paired-_t_-test). PowerPoint slide
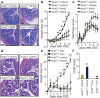
Figure 2. Rescue of severe colitis with nicotinamide or tryptophan di-peptides.
a, Colon histopathology (haematoxylin and eosin, day 10 after DSS challenge; scale bars, 100 μm), b, percentage weight loss, and c, diarrhoea scores of DSS-treated Ace2 +/y and Ace2 −/y littermates that received vehicle or nicotinamide (NAM) in their drinking water. Nicotinamide treatment was started 3 days before DSS challenge. d, Colon histopathology (haematoxylin and eosin, day 7; scale bars, 100 μm), e, percentage weight loss, and f, crypt injury scores of Ace2 +/y and Ace2 −/y mice fed a di-peptidic tryptophan diet (Trp+) or normal chow (Control). Values are mean ± s.e.m. of 3–10 mice per group. *P < 0.05, **P < 0.01 comparing Ace2 −/y mice on a normal diet with those on Trp+ diet, or vehicle- versus nicotinamide-treated Ace2 −/y mice. ##P < 0.01 comparing Ace2 +/y versus Ace2 −/y mice (paired-_t_-test). PowerPoint slide
Figure 3. Tryptophan controls antimicrobial peptides and mTOR activity.
a, b, mRNA expression levels of antimicrobial peptides in epithelial cells isolated from the small intestine of a, unchallenged Ace2 +/y and Ace2 −/y littermates, and b, Ace2 +/y mice fed a tryptophan-free diet (Trp−) or normal chow (Control). c, mRNA expression levels of antimicrobial peptide Defa1 in Ace2 +/y and Ace2 −/y littermates fed a Trp+ diet or normal chow (Control) for 10 days. d, e, Immunohistochemistry to detect levels of phosphorylated S6 (brown) in the small intestine of d, unchallenged Ace2 +/y and Ace2 −/y littermates or e, Ace2 −/y mice fed a Trp+ or normal chow diet (Control). Scale bars, 200 μm. f, Colon histopathology (haematoxylin and eosin, day 8; scale bars, 100 μm) of DSS treated wild-type mice receiving vehicle or rapamycin (RAPA) i.p., initiated 6 days before DSS challenge. Values are mean ± s.e.m. of 5–6 mice per group. *P < 0.05, **P < 0.01 comparing Ace2 +/y with Ace2 −/y mice; #P < 0.05, ##P < 0.01 comparing Ace2 +/y mice on normal diet with those on Trp− diet (paired-_t_-test). PowerPoint slide
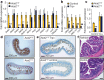
Figure 4. Altered gut bacteria from Ace2 mutant mice can confer susceptibility to colitis.
a, b, Principal coordinate analysis plots; a, calculated by Bray–Curtis algorithm and b, based on unweighted UniFrac analysis. Plots show the similarity among ileocaecal bacterial communities in Ace2 +/y and Ace2 −/y mice fed a Trp+ diet or normal chow (Control) for 10 days. Only the two axes with high _R_2 values are shown (axis 1, _R_2 = 0.335; axis 2, _R_2 = 0.8116). Each dot represents data from an individual animal. c, Comparison of microbial communities in Ace2 +/y and Ace2 −/y mice fed a Trp+ diet or normal chow (Control). The heat map depicts abundance of the top 25 species level OTUs contributing significantly to the axis shown in the weighted principal coordinate analysis plot (a). d, Diarrhoea scores and e, colon histopathology (haematoxylin and eosin, day 7; scale bars, 100 μm) of DSS challenged germ-free mice that received intestinal microbiota from Ace2 +/y or Ace2 −/y littermates. Values are mean ± s.e.m. of 4–6 mice per group. *P < 0.05 (paired-_t_-test). PowerPoint slide
Comment in
- Immunology: Malnutrition promotes rogue bacteria.
Izcue A, Powrie F. Izcue A, et al. Nature. 2012 Jul 25;487(7408):437-9. doi: 10.1038/487437a. Nature. 2012. PMID: 22836994 No abstract available. - Inflammation: Colitis, microbiota and malnutrition.
Ray K. Ray K. Nat Rev Gastroenterol Hepatol. 2012 Sep;9(9):490. doi: 10.1038/nrgastro.2012.151. Epub 2012 Aug 7. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22868660 No abstract available.
Similar articles
- ACE2 - from the renin-angiotensin system to gut microbiota and malnutrition.
Perlot T, Penninger JM. Perlot T, et al. Microbes Infect. 2013 Nov;15(13):866-73. doi: 10.1016/j.micinf.2013.08.003. Epub 2013 Aug 17. Microbes Infect. 2013. PMID: 23962453 Free PMC article. Review. - Immunology: Malnutrition promotes rogue bacteria.
Izcue A, Powrie F. Izcue A, et al. Nature. 2012 Jul 25;487(7408):437-9. doi: 10.1038/487437a. Nature. 2012. PMID: 22836994 No abstract available. - ACE2 shedding exacerbates sepsis-induced gut leak via loss of microbial metabolite 5-methoxytryptophan.
Gong J, Lu H, Li Y, Xu Q, Ma Y, Lou A, Cui W, Song W, Qu P, Chen Z, Quan L, Liu X, Meng Y, Li X. Gong J, et al. Microbiome. 2025 May 29;13(1):136. doi: 10.1186/s40168-025-02128-4. Microbiome. 2025. PMID: 40442816 Free PMC article. - Protein Malnutrition Alters Tryptophan and Angiotensin-Converting Enzyme 2 Homeostasis and Adaptive Immune Responses in Human Rotavirus-Infected Gnotobiotic Pigs with Human Infant Fecal Microbiota Transplant.
Fischer DD, Kandasamy S, Paim FC, Langel SN, Alhamo MA, Shao L, Chepngeno J, Miyazaki A, Huang HC, Kumar A, Rajashekara G, Saif LJ, Vlasova AN. Fischer DD, et al. Clin Vaccine Immunol. 2017 Aug 4;24(8):e00172-17. doi: 10.1128/CVI.00172-17. Print 2017 Aug. Clin Vaccine Immunol. 2017. PMID: 28637803 Free PMC article. - Angiotensin-converting enzyme 2 in acute respiratory distress syndrome.
Imai Y, Kuba K, Penninger JM. Imai Y, et al. Cell Mol Life Sci. 2007 Aug;64(15):2006-12. doi: 10.1007/s00018-007-6228-6. Cell Mol Life Sci. 2007. PMID: 17558469 Free PMC article. Review.
Cited by
- Towards a more comprehensive concept for prebiotics.
Bindels LB, Delzenne NM, Cani PD, Walter J. Bindels LB, et al. Nat Rev Gastroenterol Hepatol. 2015 May;12(5):303-10. doi: 10.1038/nrgastro.2015.47. Epub 2015 Mar 31. Nat Rev Gastroenterol Hepatol. 2015. PMID: 25824997 Review. - The Impact of Angiotensin-Converting Enzyme-2/Angiotensin 1-7 Axis in Establishing Severe COVID-19 Consequences.
Maranduca MA, Tanase DM, Cozma CT, Dima N, Clim A, Pinzariu AC, Serban DN, Serban IL. Maranduca MA, et al. Pharmaceutics. 2022 Sep 8;14(9):1906. doi: 10.3390/pharmaceutics14091906. Pharmaceutics. 2022. PMID: 36145655 Free PMC article. Review. - COVID-19 and the Gut Microbiome: More than a Gut Feeling.
van der Lelie D, Taghavi S. van der Lelie D, et al. mSystems. 2020 Jul 21;5(4):e00453-20. doi: 10.1128/mSystems.00453-20. mSystems. 2020. PMID: 32694127 Free PMC article. - Impaired tryptophan metabolism in the gastrointestinal tract of patients with critical coronavirus disease 2019.
Yokoyama Y, Ichiki T, Yamakawa T, Tsuji Y, Kuronuma K, Takahashi S, Narimatsu E, Nakase H. Yokoyama Y, et al. Front Med (Lausanne). 2022 Aug 10;9:941422. doi: 10.3389/fmed.2022.941422. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36035409 Free PMC article. - Probiotic Chocolate Containing Lactobacillus plantarum Dad-13 Alters the Gut Microbiota Composition of Undernourished Children in Lombok: A Randomized Double-Blind Trial.
Rahayu ES, Yoga WK, Komalasari H, Mariyatun M, Yuda WA, Manurung NEP, Hasan PN, Suharman S, Pamungkaningtyas FH, Nurfiana DA, Pramesi PC, Gatya M, Therdtatha P, Nakayama J, Juffrie M, Djaafar TF, Marwati T, Utami T. Rahayu ES, et al. Int J Food Sci. 2024 Aug 3;2024:9493797. doi: 10.1155/2024/9493797. eCollection 2024. Int J Food Sci. 2024. PMID: 39132547 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous