Porphyromonas gingivalis mediates inflammasome repression in polymicrobial cultures through a novel mechanism involving reduced endocytosis - PubMed (original) (raw)
Porphyromonas gingivalis mediates inflammasome repression in polymicrobial cultures through a novel mechanism involving reduced endocytosis
Debra J Taxman et al. J Biol Chem. 2012.
Abstract
The interleukin (IL)-1β-processing inflammasome has recently been identified as a target for pathogenic evasion of the inflammatory response by a number of bacteria and viruses. We postulated that the periodontal pathogen, Porphyromonas gingivalis may suppress the inflammasome as a mechanism for its low immunogenicity and pathogenic synergy with other, more highly immunogenic periodontal bacteria. Our results show that P. gingivalis lacks signaling capability for the activation of the inflammasome in mouse macrophages. Furthermore, P. gingivalis can suppress inflammasome activation by another periodontal bacterium, Fusobacterium nucleatum. This repression affects IL-1β processing, as well as other inflammasome-mediated processes, including IL-18 processing and cell death, in both human and mouse macrophages. F. nucleatum activates IL-1β processing through the Nlrp3 inflammasome; however, P. gingivalis repression is not mediated through reduced levels of inflammasome components. P. gingivalis can repress Nlrp3 inflammasome activation by Escherichia coli, and by danger-associated molecular patterns and pattern-associated molecular patterns that mediate activation through endocytosis. However, P. gingivalis does not suppress Nlrp3 inflammasome activation by ATP or nigericin. This suggests that P. gingivalis may preferentially suppress endocytic pathways toward inflammasome activation. To directly test whether P. gingivalis infection affects endocytosis, we assessed the uptake of fluorescent particles in the presence or absence of P. gingivalis. Our results show that P. gingivalis limits both the number of cells taking up beads and the number of beads taken up for bead-positive cells. These results provide a novel mechanism of pathogen-mediated inflammasome inhibition through the suppression of endocytosis.
Figures
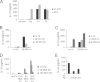
FIGURE 1.
P. gingivalis lacks second signal activation ability for IL-1β production and can repress second signal activation by F. nucleatum. ELISAs of IL-1β in cell supernatants were performed following 12–16-h infection. A, mouse BMDM were infected with 20 m.o.i. P. gingivalis (Pg), F. nucleatum (Fn), or E. coli (Ec). Where indicated, cells were treated for 30 min with 2 m
m
ATP immediately prior to collection of supernatants. B, BMDM were infected with Fn and Pg alone or in combination as indicated. C, BMDM were infected with Fn and Ec alone or in combination as indicated. D, BMDM were treated with a range of doses of Fn and Pg. E, BMDM were treated with Fn and Pg strains A7436 or 381. Results represent the averages and standard deviation of duplicates and are representative of at least three independent experiments.
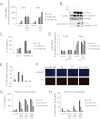
FIGURE 2.
Pg represses inflammasome activation by Fn. A, real-time PCR of Il1b mRNA in mouse BMDM was performed 2 or 5 h following infection with a range of doses of Fn and Pg. Expression is normalized to the expression of 18 S rRNA and standardized to 1 in uninfected cells. Results represent the average ±S.D. (error bars) of biological triplicates and are representative of three independent experiments. B, Western analysis is shown for pro-IL-1β and pro-caspase-1 protein in cell lysates and cleaved (activated) IL-1β and caspase-1 p10 in cell supernatants 14 h following infection with 20 m.o.i. Fn and 100 m.o.i. Pg. GAPDH is shown as a loading control. Results are representative of three independent experiments. C, ELISA of IL-18 expression in BMDM is shown following 14-h infection with Fn and Pg at indicated m.o.i. Results represent the averages ±S.D. of duplicates and are representative of at least three independent experiments. D, real-time PCR of Il18 mRNA in BMDM was performed 2 or 5 h following infection with a range of dose of Fn and Pg. Expression is normalized to the expression of 18 S rRNA and standardized to 1 in uninfected cells. Results represent the averages ±S.D. of biological triplicates and are representative of three independent experiments. E, percentage of cell death in BMDM was determined by ToxiLight® assay 16 h following infection with 20 m.o.i. Fn alone or together with (+) 12.5, (++) 50, or (+++) 200 m.o.i. Pg. Results are representative of three independent experiments. F, propidium iodide (P. I.) stain is shown as an indicator of cell death in BMDM 16 h following infection with 20 m.o.i. Fn and 100 m.o.i. Pg. Hoechst staining is shown as a control. Results are representative of two independent experiments. G, ELISA of IL-1β secretion in human macrophages is shown 14 h following infection with Fn and Pg at a range of m.o.i. Results represent the averages ±S.D. of duplicates and are representative of two independent experiments. H, percentage of cell death in human macrophages was determined by ToxiLight® assay 16 h following infection with Fn and Pg at a range of m.o.i.
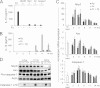
FIGURE 3.
Pg does not repress the inflammasome by ablating the expression of its protein components. A, ELISA of IL-1β secretion is shown for BMDM isolated from wild type (WT) and gene-deletion mice 16 h following infection with Fn. B, ELISA of IL-1β secretion in wild type mouse BMDM is shown following a time course of infection with 20 m.o.i. Fn and 100 m.o.i. Pg alone or in combination. C, real-time PCR of Nlrp3, Asc, and caspase-1 mRNA in BMDM following a time course of infection with 20 m.o.i. Fn and 100 m.o.i. Pg is shown. Expression is normalized to the expression of 18 S rRNA and standardized to 1 in uninfected cells. Results represent the average ±S.D. (error bars) of biological triplicates and are representative of three independent experiments. D, Western analysis was performed for Nlrp3, Asc, and caspase-1 protein levels in BMDM 8, 11, and 14 h following infection with 20 m.o.i. Fn and 100 m.o.i. Pg. Actin is shown as a loading control, and cleaved caspase-1 in cell supernatants is provided for reference. Results are representative of three independent experiments.
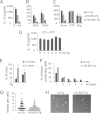
FIGURE 4.
Pg inflammasome repression is mediated through blockade of endocytosis. ELISA of mouse BMDM demonstrates that Pg can repress the activation of IL-1β by (A) E. coli; by (B) LPS plus MSU or PGN; and by (C) LPS plus alum, but not LPS plus ATP or nigericin (Nig). Results are representative of three independent experiments. D, IL-1β ELISA of BMDM treated with LPS plus ATP is shown. Pg was added over a time course preceding the ATP treatment. Representative of two independent experiments. E, percentages of BMDM uptaking beads following 2-h exposure are shown. Cells were pretreated with 100 m.o.i. Pg where indicated. F, numbers of beads in the bead-containing cell population for uninfected cells and cells infected with 100 m.o.i. Pg are shown. Numbers are expressed as a percentage of the total bead-containing cells. G, a diagrammatic representation of the number of beads per cell for uninfected cells and cells infected with 100 m.o.i. Pg is shown. Each cell is represented as a dot. H, a representative confocal microscopy image for bead uptake in uninfected cells and cells infected with 100 m.o.i. Pg is provided. The number of internalized beads was counted in >100 cells per each of three independent experiments.
Similar articles
- Activation of NLRP3 and AIM2 inflammasomes by Porphyromonas gingivalis infection.
Park E, Na HS, Song YR, Shin SY, Kim YM, Chung J. Park E, et al. Infect Immun. 2014 Jan;82(1):112-23. doi: 10.1128/IAI.00862-13. Epub 2013 Oct 14. Infect Immun. 2014. PMID: 24126516 Free PMC article. - Porphyromonas gingivalis triggers NLRP3-mediated inflammasome activation in macrophages in a bacterial gingipains-independent manner.
Okano T, Ashida H, Suzuki S, Shoji M, Nakayama K, Suzuki T. Okano T, et al. Eur J Immunol. 2018 Dec;48(12):1965-1974. doi: 10.1002/eji.201847658. Epub 2018 Oct 22. Eur J Immunol. 2018. PMID: 30280383 - Down-regulation of NLRP3 inflammasome in gingival fibroblasts by subgingival biofilms: involvement of Porphyromonas gingivalis.
Belibasakis GN, Guggenheim B, Bostanci N. Belibasakis GN, et al. Innate Immun. 2013 Feb;19(1):3-9. doi: 10.1177/1753425912444767. Epub 2012 Apr 20. Innate Immun. 2013. PMID: 22522430 - Historical aspects of studies on roles of the inflammasome in the pathogenesis of periodontal diseases.
Shibata K. Shibata K. Mol Oral Microbiol. 2018 Jun;33(3):203-211. doi: 10.1111/omi.12217. Epub 2018 Feb 20. Mol Oral Microbiol. 2018. PMID: 29360244 Review. - Immunological Pathways Triggered by Porphyromonas gingivalis and Fusobacterium nucleatum: Therapeutic Possibilities?
de Andrade KQ, Almeida-da-Silva CLC, Coutinho-Silva R. de Andrade KQ, et al. Mediators Inflamm. 2019 Jun 24;2019:7241312. doi: 10.1155/2019/7241312. eCollection 2019. Mediators Inflamm. 2019. PMID: 31341421 Free PMC article. Review.
Cited by
- Bacterial subversion of NLR-mediated immune responses.
Kienes I, Johnston EL, Bitto NJ, Kaparakis-Liaskos M, Kufer TA. Kienes I, et al. Front Immunol. 2022 Jul 28;13:930882. doi: 10.3389/fimmu.2022.930882. eCollection 2022. Front Immunol. 2022. PMID: 35967403 Free PMC article. Review. - Social networking at the microbiome-host interface.
Lamont RJ, Hajishengallis G, Koo H. Lamont RJ, et al. Infect Immun. 2023 Sep 14;91(9):e0012423. doi: 10.1128/iai.00124-23. Epub 2023 Aug 18. Infect Immun. 2023. PMID: 37594277 Free PMC article. Review. - Distinct lipid a moieties contribute to pathogen-induced site-specific vascular inflammation.
Slocum C, Coats SR, Hua N, Kramer C, Papadopoulos G, Weinberg EO, Gudino CV, Hamilton JA, Darveau RP, Genco CA. Slocum C, et al. PLoS Pathog. 2014 Jul 10;10(7):e1004215. doi: 10.1371/journal.ppat.1004215. eCollection 2014 Jul. PLoS Pathog. 2014. PMID: 25010102 Free PMC article. - Oral pathogen aggravates atherosclerosis by inducing smooth muscle cell apoptosis and repressing macrophage efferocytosis.
Xie H, Qin Z, Ling Z, Ge X, Zhang H, Guo S, Liu L, Zheng K, Jiang H, Xu R. Xie H, et al. Int J Oral Sci. 2023 Jun 28;15(1):26. doi: 10.1038/s41368-023-00232-5. Int J Oral Sci. 2023. PMID: 37380627 Free PMC article. - Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action.
Hayward JA, Mathur A, Ngo C, Man SM. Hayward JA, et al. Microbiol Mol Biol Rev. 2018 Sep 12;82(4):e00015-18. doi: 10.1128/MMBR.00015-18. Print 2018 Dec. Microbiol Mol Biol Rev. 2018. PMID: 30209070 Free PMC article. Review.
References
- Dinarello C. A. (1998) Interleukin-1β, interleukin-18, and the interleukin-1β-converting enzyme. Ann. N.Y. Acad. Sci. 856, 1–11 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007001/AI/NIAID NIH HHS/United States
- R01 AI088255/AI/NIAID NIH HHS/United States
- R56 AI029564/AI/NIAID NIH HHS/United States
- AI029564/AI/NIAID NIH HHS/United States
- R01 AI029564/AI/NIAID NIH HHS/United States
- DE016326/DE/NIDCR NIH HHS/United States
- AI088255/AI/NIAID NIH HHS/United States
- U54 AI057157/AI/NIAID NIH HHS/United States
- T32AI007001/AI/NIAID NIH HHS/United States
- AI057157/AI/NIAID NIH HHS/United States
- R37 AI029564/AI/NIAID NIH HHS/United States
- R01 DE016326/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous