Effects of oxygen-glucose deprivation on microglial mobility and viability in developing mouse hippocampal tissues - PubMed (original) (raw)
Effects of oxygen-glucose deprivation on microglial mobility and viability in developing mouse hippocampal tissues
Ukpong Eyo et al. Glia. 2012 Nov.
Abstract
As brain-resident immune cells, microglia (MG) survey the brain parenchyma to maintain homeostasis during development and following injury. Research in perinatal stroke, a leading cause of lifelong disability, has implicated MG as targets for therapeutic intervention during stroke. Although MG responses are complex, work in developing rodents suggests that MG limit brain damage after stroke. However, little is known about how energy-limiting conditions affect MG survival and mobility (motility and migration) in developing brain tissues. Here, we used confocal time-lapse imaging to monitor MG viability and mobility during hypoxia or oxygen-glucose deprivation (OGD) in hippocampal tissue slices derived from neonatal GFP-reporter mice (CX3CR1(GFP/+) ). We found that MG remain viable for at least 6 h of hypoxia but begin to die after 2 h of OGD, while both hypoxia and OGD reduce MG motility. Unexpectedly, some MG retain or recover motility during OGD and can engulf dead cells. Additionally, MG from younger neonates (P2-P3) are more resistant to OGD than those from older ones (P6-P7), indicating increasing vulnerability with developmental age. Finally, transient (2 h) OGD also increases MG death, and although motility is rapidly restored after transient OGD, it remains below control levels for many hours. Together, these results show that MG in neonatal mouse brain tissues are vulnerable to both transient and sustained OGD, and many MG die within hours after onset of OGD. Preventing MG death may, therefore, provide a strategy for promoting tissue restoration after stroke.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
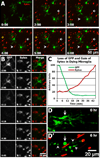
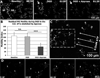
Figure 1. OGD induces microglial cell death in acutely isolated neonatal hippocampal slices
A, Time-lapse imaging shows that many non-microglial cells die under control conditions, as shown by Sytox labeling of nuclei of non-GFP expressing cells. Several dying cells are evident within the first two hours after tissue excision. More non-microglial cells die during the next several hours (arrows and arrowhead), but no GFP-expressing microglia die (assessed by loss of GFP and gain of Sytox) during this period of time. B, Images from a time-lapse movie of a microglial cell that dies during OGD. The microglial cell loses GFP and within minutes becomes labeled with Sytox. Note that the cell loses GFP before showing obvious signs of blebbing. See Supplemental Movie 1. C, Quantification shows that, for the dying microglia in (B), the GFP signal rapidly decreases in the soma as Sytox signal subsequently increases in the nucleus. D-D’, Some microglia become unhealthy looking after prolonged OGD (D’), showing rounded soma (arrow) and beaded branches (arrowheads), but retain GFP and fail to take up Sytox (red).
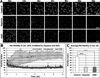
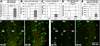
Figure 2. OGD, but not hypoxia, induces microglial cell death in acutely isolated neonatal hippocampal slices
A, Representative images of slices with GFP-expressing microglia at the beginning and end of six hour long imaging experiments under control (left), hypoxia (middle), and OGD (right) conditions. All microglia under control conditions remain viable during this time (assessed by retention of GFP). Under hypoxia, only one microglia cell in the field of view (arrow) dies, as assessed by loss of GFP (arrowhead). In contrast, several microglia die during 6hr of sustained OGD (arrows and arrowheads), and some of the surviving microglia have rounded somata (dashed circles). B, The percent of dying microglia was determined by the method in (A) for control, hypoxia, and OGD conditions. No microglial cell death was observed in control slices. Microglial cell death increased significantly during OGD but not hypoxia. C, Graph shows a cumulative increase in microglial cell death during 6 hours of sustained OGD. D, Graph shows average number of microglia that die in each field of view per hour of OGD. Significance in C-D is shown relative to the first hour. *P ≤ 0.05; ***P ≤ 0.000005
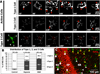
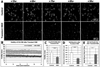
Figure 3. Both hypoxia and OGD reduce overall microglial process motility in stratum radiatum of hippocampal area CA1
A, Representative difference images showing microglial motility in the CA1 stratum radiatum during control (top), hypoxia (middle), or OGD (bottom). Microglia maintain their process motility under control conditions, but loss of signal in these difference images shows that motility is substantially reduced under both hypoxic and OGD conditions. See Supplementary Movie 2. B, Quantitative analysis of motility (motility index) of microglia in several hippocampal slices per condition shows an increasing and sustained high level of motility in control condition but rapid and persistent depression of microglia motility in hypoxia- and OGD-treated slices. C, The average motility index over the 6 hr of treatment was significantly reduced in both the hypoxic and OGD groups relative to control. ***P ≤ 0.000005
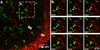
Figure 4. Microglial motility can persist or spontaneously recover under hypoxia or OGD
A, Difference images showing individual microglial cells that were persistently motile (Type 1 cells), transiently immotile (Type 2 cells), or persistently immotile (Type 3 cells). Type 1 and Type 2 microglial cells showed high levels of motility around the edges of cell body and processes (red arrows). Conversely, Type 3 cells retained their branch processes (numbered in the raw image), and the position and shape of the soma was essentially unchanged (dashed red lines). B, Graph showing the distribution of Type 1, 2, and 3 microglial cells after 6 hr in control, hypoxia, or OGD conditions. The percent of Type 1 (persistently motile) cells was significantly reduced during hypoxia and OGD. Type 3 (immotile) cells were never observed during control conditions. The total number of cells analyzed per condition is shown above each bar. C, Image of a slice during hypoxia with microglia (green) and Sytox-labeled dead cells (red) showing representative distribution of Type 1 (white arrows), Type 2 (white arrowheads), and Type 3 (yellow arrowheads) microglial cells. Note that many of the active microglial cells (Types 1 and 2) are found in or near the neuron-populated SP. SO: stratum oriens; SP: stratum pyramidale; SR: stratum radiatum.
Figure 5. OGD-resistant microglia can contact and phagocytize dead cells during OGD
A, Hippocampal slice during OGD showing MG (green) amongst Sytox-labeled dead cells (red) in the stratum radiatum (SR) and stratum pyramidale (SP). B, Time-lapse sequence of boxed region in (A). A Sytox-labeled dead cell (arrow) is contacted and eventually engulfed by a nearby type 2 MG cell during continuous OGD. Images in A and B are projection images of 6 image planes (18 µm deep). Time is shown in hr:min. See Supplemental Movie 3.
Figure 6. Developmental sensitivity of microglial viability and motility in neonatal hippocampal slices
Hippocampal slices from younger (P2/P3) or older (P6/P7) neonates were imaged for 6 hours during OGD. A–B, Quantitative analysis showed that microglia in the younger tissues were much less likely to die (A) and survived longer (B) under OGD. C, The fraction of microglia that were motile during OGD was much higher in the younger tissues. D-E, The most active microglia during OGD had a higher peak velocity (D) and travelled farther (E) in the younger tissues. F-G, Two representative examples of the most active microglial cells showing migration tracks (colored lines in 6 hr images) during sustained OGD in a P2 (F) and P6 (G) tissue slice. Arrowheads in G indicate four microglial cells that died during the imaging session. None of the microglia in this field of view died in the P2 slice. Time is shown as hr:min. See Supplemental Movie 4. SO: stratum oriens; SP: stratum pyramidale; SR: stratum radiatum.
Figure 7. Motile and immotile microglia during transient (2hr) OGD
Microglial motility was monitored for 1 hr before, 2 hr during, and 1 hr after transient OGD (tOGD). A, Representative image of area CA1 in a P6 hippocampal slice subjected to tOGD. B, Top Row: Difference images of a Type 1 microglial cell in the SP (top box in Panel A) showing that motility is maintained during OGD. Bottom Row: Difference images of a representative Type 3 cell in the SR showing inhibition of motility during tOGD but rapid recovery of motility following washout. C, The motility indices of several cells that displayed motile (n=12) or immotile (n=12) phenotypes were averaged. Note that immotile cells started with a lower baseline motility level, were rapidly inhibited by OGD, but recovered motility immediately after OGD washout. D, The average motility was calculated for the baseline, OGD, and washout periods. Motility for ‘immotile cells’ was significantly depressed during OGD and enhanced immediately after OGD. Cell motility for persistently ‘motile cells’ did not change significantly throughout the observation period. See Supplemental Movie 5. ***P ≤ 0.000005. SP: stratum pyramidale; SR: stratum radiatum.
Figure 8. Transient (2hr) OGD caused a prolonged alteration of microglia behavior
Multiple slices from the same animals were exposed to either control conditions or transient OGD for 2 hr, returned to normoxic/normoglycemic conditions for 4 hr, then mounted and imaged simultaneously at 3 min intervals for several hours. This serves as an extended ex vivo “reperfusion” model. A, Representative time-lapse sequences (difference images) during the simulated reperfusion period from two daughter slices previously subjected to control conditions (top row) or tOGD (bottom row). Time is shown as hours after end of 2hr of OGD. B–C, Quantification of microglia motility shows a persistent and statistically significant reduction in microglial motility during the washout period following OGD. D, Microglial migration distance was significantly reduced during the 6 hr period following 2 hr tOGD. F, There was a significant increase in the number of MG that died in the 6 hr period following 2 hr tOGD. ***P ≤ 0.0005
Figure 9
Similar articles
- P2X7 receptor activation regulates microglial cell death during oxygen-glucose deprivation.
Eyo UB, Miner SA, Ahlers KE, Wu LJ, Dailey ME. Eyo UB, et al. Neuropharmacology. 2013 Oct;73:311-9. doi: 10.1016/j.neuropharm.2013.05.032. Epub 2013 Jun 12. Neuropharmacology. 2013. PMID: 23770338 Free PMC article. - Microglia and macrophages differentially modulate cell death after brain injury caused by oxygen-glucose deprivation in organotypic brain slices.
Girard S, Brough D, Lopez-Castejon G, Giles J, Rothwell NJ, Allan SM. Girard S, et al. Glia. 2013 May;61(5):813-24. doi: 10.1002/glia.22478. Epub 2013 Feb 13. Glia. 2013. PMID: 23404620 Free PMC article. - Neuroprotective effects of the anti-inflammatory compound triflusal on ischemia-like neurodegeneration in mouse hippocampal slice cultures occur independent of microglia.
Montero Domínguez M, González B, Zimmer J. Montero Domínguez M, et al. Exp Neurol. 2009 Jul;218(1):11-23. doi: 10.1016/j.expneurol.2009.03.023. Epub 2009 Mar 31. Exp Neurol. 2009. PMID: 19341733 - Oxygen/glucose deprivation in hippocampal slices: altered intraneuronal elemental composition predicts structural and functional damage.
Taylor CP, Weber ML, Gaughan CL, Lehning EJ, LoPachin RM. Taylor CP, et al. J Neurosci. 1999 Jan 15;19(2):619-29. doi: 10.1523/JNEUROSCI.19-02-00619.1999. J Neurosci. 1999. PMID: 9880582 Free PMC article. - Immunotoxic depletion of microglia in mouse hippocampal slice cultures enhances ischemia-like neurodegeneration.
Montero M, González B, Zimmer J. Montero M, et al. Brain Res. 2009 Sep 29;1291:140-52. doi: 10.1016/j.brainres.2009.06.097. Epub 2009 Jul 10. Brain Res. 2009. PMID: 19595678
Cited by
- P2X7 receptor activation regulates microglial cell death during oxygen-glucose deprivation.
Eyo UB, Miner SA, Ahlers KE, Wu LJ, Dailey ME. Eyo UB, et al. Neuropharmacology. 2013 Oct;73:311-9. doi: 10.1016/j.neuropharm.2013.05.032. Epub 2013 Jun 12. Neuropharmacology. 2013. PMID: 23770338 Free PMC article. - Microglial Function during Glucose Deprivation: Inflammatory and Neuropsychiatric Implications.
Churchward MA, Tchir DR, Todd KG. Churchward MA, et al. Mol Neurobiol. 2018 Feb;55(2):1477-1487. doi: 10.1007/s12035-017-0422-9. Epub 2017 Feb 7. Mol Neurobiol. 2018. PMID: 28176274 Free PMC article. - The role of microglia in neuronal and cognitive function during high altitude acclimatization.
Hatch K, Lischka F, Wang M, Xu X, Stimpson CD, Barvir T, Cramer NP, Perl DP, Yu G, Browne CA, Dickstein DL, Galdzicki Z. Hatch K, et al. Sci Rep. 2024 Aug 16;14(1):18981. doi: 10.1038/s41598-024-69694-9. Sci Rep. 2024. PMID: 39152179 Free PMC article. - Abscopal Activation of Microglia in Embryonic Fish Brain Following Targeted Irradiation with Heavy-Ion Microbeam.
Yasuda T, Kamahori M, Nagata K, Watanabe-Asaka T, Suzuki M, Funayama T, Mitani H, Oda S. Yasuda T, et al. Int J Mol Sci. 2017 Jul 4;18(7):1428. doi: 10.3390/ijms18071428. Int J Mol Sci. 2017. PMID: 28677658 Free PMC article. - Xanomeline Protects Cortical Cells From Oxygen-Glucose Deprivation via Inhibiting Oxidative Stress and Apoptosis.
Xin R, Chen Z, Fu J, Shen F, Zhu Q, Huang F. Xin R, et al. Front Physiol. 2020 Jun 12;11:656. doi: 10.3389/fphys.2020.00656. eCollection 2020. Front Physiol. 2020. PMID: 32595528 Free PMC article.
References
- Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–324. - PubMed
- Carlsson Y, Schwendimann L, Vontell R, Rousset CI, Wang X, Lebon S, Charriaut-Marlangue C, Supramaniam V, Hagberg H, Gressens P, Jacotot E. Genetic inhibition of caspase-2 reduces hypoxic-ischemic and excitotoxic neonatal brain injury. Ann Neurol. 2011;70:781–789. - PubMed
- Cavaliere F, Dinkel K, Reymann K. Microglia response and P2 receptor participation in oxygen/glucose deprivation-induced cortical damage. Neuroscience. 2005;136:615–623. - PubMed
- Cavaliere F, Florenzano F, Amadio S, Fusco FR, Viscomi MT, D'Ambrosi N, Vacca F, Sancesario G, Bernardi G, Molinari M, Volonte C. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120:85–98. - PubMed
- Chock VY, Giffard RG. Development of neonatal murine microglia in vitro: changes in response to lipopolysaccharide and ischemia-like injury. Pediatr Res. 2005;57:475–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 RR017941/RR/NCRR NIH HHS/United States
- P30 DC010362/DC/NIDCD NIH HHS/United States
- NS064006/NS/NINDS NIH HHS/United States
- R21 AA018823/AA/NIAAA NIH HHS/United States
- R01 NS043468/NS/NINDS NIH HHS/United States
- DC010362/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials