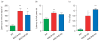Monoamine oxidase A and A/B knockout mice display autistic-like features - PubMed (original) (raw)
Monoamine oxidase A and A/B knockout mice display autistic-like features
Marco Bortolato et al. Int J Neuropsychopharmacol. 2013 May.
Abstract
Converging lines of evidence show that a sizable subset of autism-spectrum disorders (ASDs) is characterized by increased blood levels of serotonin (5-hydroxytryptamine, 5-HT), yet the mechanistic link between these two phenomena remains unclear. The enzymatic degradation of brain 5-HT is mainly mediated by monoamine oxidase (MAO)A and, in the absence of this enzyme, by its cognate isoenzyme MAOB. MAOA and A/B knockout (KO) mice display high 5-HT levels, particularly during early developmental stages. Here we show that both mutant lines exhibit numerous behavioural hallmarks of ASDs, such as social and communication impairments, perseverative and stereotypical responses, behavioural inflexibility, as well as subtle tactile and motor deficits. Furthermore, both MAOA and A/B KO mice displayed neuropathological alterations reminiscent of typical ASD features, including reduced thickness of the corpus callosum, increased dendritic arborization of pyramidal neurons in the prefrontal cortex and disrupted microarchitecture of the cerebellum. The severity of repetitive responses and neuropathological aberrances was generally greater in MAOA/B KO animals. These findings suggest that the neurochemical imbalances induced by MAOA deficiency (either by itself or in conjunction with lack of MAOB) may result in an array of abnormalities similar to those observed in ASDs. Thus, MAOA and A/B KO mice may afford valuable models to help elucidate the neurobiological bases of these disorders and related neurodevelopmental problems.
Conflict of interest statement
Statement of Interest
None.
Figures
Fig. 1
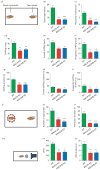
Monoamine oxidase (MAO)A knockout (KO) and MAOA/B KO mice display social and communication impairments. (a) Schematic diagram of the social interaction paradigm. (b, c) MAOA- and MAOA/B-deficient mice exhibit a significant reduction in social approaches and reciprocal interactions towards the foreign conspecifics compared to wild-type (WT) mice. (d_–_i) MAO mutant genotypes also displayed a marked decrease in overall sniffing behaviour in both number and duration in comparison to their WT counterparts. While this decrease was most pronounced in anogenital sniffing, MAOA/B-deficient mice also spent significantly less time in frontal sniffing than WT animals. (j_–_l) In the social investigation paradigm, both MAO mutant genotypes displayed a significant decrease in number and duration of social approaches. (m_–_p) Maternal separation-induced ultrasonic vocalizations (USVs) were markedly lower in MAOA KO and MAOA/B KO pups than WT pups. Values are displayed as means±S.E.M. *p<0.05, **p<0.01 and ***p<0.001 compared to WT mice. For social interactions _n_=7–10; social investigation _n_=8–10; maternal separation-induced USVs _n_=13–20.
Fig. 2
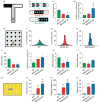
Monoamine oxidase (MAO)A knockout (KO) and MAOA/B KO mice exhibit perseverative behavioural responses. (a) Schematic representation of the T-maze paradigm. (b) Example of a typical set of alternations for each genotype where the change from black (left arm) to white (right arm) or vice versa indicates an alternation. (c, d) While both MAOA and MAOA/B KO mice displayed a significant reduction in spontaneous alternations compared to wild-type (WT) mice, MAOA/B-deficient mice engaged in a higher number of repetitive arm entries prior to the first alternation compared to the other genotypes. (e) Schematic representation of the hole-board task. (f_–_h) The 16-bar histograms depict the representative cases of percent relative dispersion of head pokes across the 16 holes of the board for each genotype. (i_–_l) Both MAO mutants had significantly fewer overall head pokes, as well as a lower average number of alternations per hole, but not head pokes per hole compared to WT mice. MAOA/B KO, but not MAOA KO mice, also exhibited a significantly higher coefficient of variation than their WT counterparts, signifying greater perseverative hole exploration. (m_–_p) In the marble-burying task, both transgenic lines displayed higher marble-burying and digging activity than WT mice; however, MAOA/B KO responses to marbles were significantly more robust than MAOA KO mice. Values are displayed as means±S.E.M. *p<0.05, **p<0.01 and ***p<0.001 compared to WT mice and #p<0.05 compared to MAOA KO mice. For spontaneous alternations in T-maze _n_=5–8; hole-board _n_=8; marble burying _n_=8–9.
Fig. 3
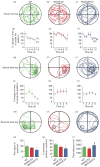
Monoamine oxidase (MAO)A/B, but not MAOA-deficient mice show a significant impairment in learning reversal. (a_–_c) Typical locomotor pathways during the initial probe trial prior to training. (d_–_i) All genotypes exhibited a significant decrease in the percent time to reach the platform for spatial learning. (j_–_o) After reversal training, only wild-type (WT) mice displayed a significant reduction in the percent time to locate the platform, signifying learning acquisition. (p_–_r) MAOA/B knockout (KO) mice showed a significant reduction in both the total duration and the percent locomotor activity in the platform-associated target quadrant compared to WT mice. This was not caused by alterations in locomotor activity as no changes were found between MAOA/B KO and WT mice. Values are displayed as means±S.E.M. *p<0.05, **p<0.01 and ***p<0.001 compared to controls. #p<0.05 compared to MAOA KO mice. † p<0.05, †† p<0.01 and ††† p<0.001 compared to first trial within subject. For reversal learning _n_=6–9.
Fig. 4
Monoamine oxidase (MAO)A knockout (KO) and MAOA/B KO mice exhibit sensory and motor alterations. (a_–_c) Both MAO mutants display a significant reduction in haptic stimulation, as well as impairments in motor function compared to wild-type (WT) counterparts in the sticky tape and the balance beam tests respectively. In comparison to WT mice, MAOA KO, but not MAOA/B KO mice show an increase in the latency to lick paws in the hot plate test, indicating thermal insensitivity. Values are displayed as means±S.E.M. *p<0.05, **p<0.01 and ***p<0.001 compared to WT mice. For sticky-tape test _n_=9–10; hot-plate _n_=9–16; balance beam _n_=10.
Fig. 5
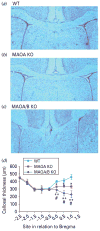
Monoamine oxidase (MAO)A knockout (KO) and MAOA/B KO mice show a reduction of callosal thickness. (a_–_c) Coronal images of the corpus callosum in wild-type (WT), MAOA KO, and MAOA/B KO mice. (d) MAOA- and MAOA/B-deficient mice show a significant reduction in thickness in the rostral level of the corpus callosum. Values are displayed as means±S.E.M. * p<0.05 and ** p<0.01 compared to WT mice and #p<0.05 compared to MAOA KO mice. For callosal thickness _n_=12–18.
Fig. 6
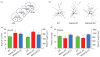
Monoamine oxidase (MAO)A knockout (KO) and MAOA/B KO mice show significant disturbances in dendritic arbourization in the orbitofrontal cortex. (a) Schematic diagram of coronal sections through mouse prefrontal cortex. The coordinates indicate position relative to bregma (Paxinos & Franklin, 2001). (b) Computer-assisted reconstructions of Golgi-stained neurons in the orbitofrontal cortex in wild-type (WT), MAOA KO and MAOA/B KO mice. Scale bar: 50 μm. (c) Both MAOA and MAOA/B KO mice display an increase in the number and length of apical dendritic branches of pyramidal neurons in the orbitofrontal cortex. (d) Conversely, MAOA KO, but not MAOA/B KO mice exhibit a significant reduction in the number and length of basilar dendritic branches in the pyramidal neurons of the orbitofrontal cortex compared to their WT counterparts. Values are displayed as means±S.E.M. *p<0.05 compared to WT mice. For dendritic analyses _n_=6–7.
Fig. 7
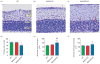
Monoamine oxidase (MAO)A/B knockout (KO) mice exhibit morphological abnormalities in the cerebellum. (a_–_c) Representative photographs of Nissl stained cerebellar layers. Cerebellar layers are labelled in yellow and red arrows represent the Purkinje cells. (d_–_f) MAOA/B KO animals have significantly fewer Purkinje cells than wild-type (WT) and MAOA KO mice. Moreover, MAOA/B KO mice show an increase in the molecular layer (ML) thickness compared to WT mice and in the granule cell layer (GCL) thickness compared to MAOA KO mice. Values are displayed as means±S.E.M. *p<0.05 and ***p<0.001 compared to WT mice. #p<0.05 and ##p<0.01 compared to MAOA KO mice. Cerebellar layers _n_=5–8. PCL, Purkinje cell layer.
Similar articles
- Social deficits and perseverative behaviors, but not overt aggression, in MAO-A hypomorphic mice.
Bortolato M, Chen K, Godar SC, Chen G, Wu W, Rebrin I, Farrell MR, Scott AL, Wellman CL, Shih JC. Bortolato M, et al. Neuropsychopharmacology. 2011 Dec;36(13):2674-88. doi: 10.1038/npp.2011.157. Epub 2011 Aug 10. Neuropsychopharmacology. 2011. PMID: 21832987 Free PMC article. - Early postnatal inhibition of serotonin synthesis results in long-term reductions of perseverative behaviors, but not aggression, in MAO A-deficient mice.
Bortolato M, Godar SC, Tambaro S, Li FG, Devoto P, Coba MP, Chen K, Shih JC. Bortolato M, et al. Neuropharmacology. 2013 Dec;75:223-32. doi: 10.1016/j.neuropharm.2013.07.003. Epub 2013 Jul 16. Neuropharmacology. 2013. PMID: 23871843 Free PMC article. - From aggression to autism: new perspectives on the behavioral sequelae of monoamine oxidase deficiency.
Bortolato M, Floris G, Shih JC. Bortolato M, et al. J Neural Transm (Vienna). 2018 Nov;125(11):1589-1599. doi: 10.1007/s00702-018-1888-y. Epub 2018 May 10. J Neural Transm (Vienna). 2018. PMID: 29748850 Free PMC article. Review. - The aggression and behavioral abnormalities associated with monoamine oxidase A deficiency are rescued by acute inhibition of serotonin reuptake.
Godar SC, Bortolato M, Castelli MP, Casti A, Casu A, Chen K, Ennas MG, Tambaro S, Shih JC. Godar SC, et al. J Psychiatr Res. 2014 Sep;56:1-9. doi: 10.1016/j.jpsychires.2014.04.014. Epub 2014 May 15. J Psychiatr Res. 2014. PMID: 24882701 Free PMC article. - Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: from neurotransmitter imbalance to impaired neurogenesis.
Naoi M, Maruyama W, Shamoto-Nagai M. Naoi M, et al. J Neural Transm (Vienna). 2018 Jan;125(1):53-66. doi: 10.1007/s00702-017-1709-8. Epub 2017 Mar 14. J Neural Transm (Vienna). 2018. PMID: 28293733 Review.
Cited by
- Contrasting characteristic behaviours among common laboratory mouse strains.
Sultana R, Ogundele OM, Lee CC. Sultana R, et al. R Soc Open Sci. 2019 Jun 12;6(6):190574. doi: 10.1098/rsos.190574. eCollection 2019 Jun. R Soc Open Sci. 2019. PMID: 31312505 Free PMC article. - Murine obscurin and Obsl1 have functionally redundant roles in sarcolemmal integrity, sarcoplasmic reticulum organization, and muscle metabolism.
Blondelle J, Marrocco V, Clark M, Desmond P, Myers S, Nguyen J, Wright M, Bremner S, Pierantozzi E, Ward S, Estève E, Sorrentino V, Ghassemian M, Lange S. Blondelle J, et al. Commun Biol. 2019 May 9;2:178. doi: 10.1038/s42003-019-0405-7. eCollection 2019. Commun Biol. 2019. PMID: 31098411 Free PMC article. - Effect of the 5-HT2C Receptor Agonist WAY-163909 on Serotonin and Dopamine Metabolism across the Rat Brain: A Quantitative and Qualitative Neurochemical Study.
Whitestone S, Deurwaerdère P, Baassiri L, Manem J, Anouar Y, Di Giovanni G, Bharatiya R, Chagraoui A. Whitestone S, et al. Int J Mol Sci. 2019 Jun 14;20(12):2925. doi: 10.3390/ijms20122925. Int J Mol Sci. 2019. PMID: 31208016 Free PMC article. - 20 ans après: a second mutation in MAOA identified by targeted high-throughput sequencing in a family with altered behavior and cognition.
Piton A, Poquet H, Redin C, Masurel A, Lauer J, Muller J, Thevenon J, Herenger Y, Chancenotte S, Bonnet M, Pinoit JM, Huet F, Thauvin-Robinet C, Jaeger AS, Le Gras S, Jost B, Gérard B, Peoc'h K, Launay JM, Faivre L, Mandel JL. Piton A, et al. Eur J Hum Genet. 2014 Jun;22(6):776-83. doi: 10.1038/ejhg.2013.243. Epub 2013 Oct 30. Eur J Hum Genet. 2014. PMID: 24169519 Free PMC article. - The role of monoamine oxidase A in aggression: Current translational developments and future challenges.
Godar SC, Fite PJ, McFarlin KM, Bortolato M. Godar SC, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2016 Aug 1;69:90-100. doi: 10.1016/j.pnpbp.2016.01.001. Epub 2016 Jan 9. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 26776902 Free PMC article. Review.
References
- Allen G, Muller RA, Courchesne E. Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biological Psychiatry. 2004;56:269–278. - PubMed
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends in Neuroscience. 2008;31:137–145. - PubMed
- Anderson GM, Freedman DX, Cohen DJ, Volkmar FR, et al. Whole blood serotonin in autistic and normal subjects. Journal of Child Psychology and Psychiatry. 1987;28:885–900. - PubMed
- Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Annals of the New York Academy of Sciences. 1990;600:331–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous