In vitro model of metastasis to bone marrow mediates prostate cancer castration resistant growth through paracrine and extracellular matrix factors - PubMed (original) (raw)
In vitro model of metastasis to bone marrow mediates prostate cancer castration resistant growth through paracrine and extracellular matrix factors
Reynald M Lescarbeau et al. PLoS One. 2012.
Abstract
The spread of prostate cancer cells to the bone marrow microenvironment and castration resistant growth are key steps in disease progression and significant sources of morbidity. However, the biological significance of mesenchymal stem cells (MSCs) and bone marrow derived extracellular matrix (BM-ECM) in this process is not fully understood. We therefore established an in vitro engineered bone marrow tissue model that incorporates hMSCs and BM-ECM to facilitate mechanistic studies of prostate cancer cell survival in androgen-depleted media in response to paracrine factors and BM-ECM. hMSC-derived paracrine factors increased LNCaP cell survival, which was in part attributed to IGFR and IL6 signaling. In addition, BM-ECM increased LNCaP and MDA-PCa-2b cell survival in androgen-depleted conditions, and induced chemoresistance and morphological changes in LNCaPs. To determine the effect of BM-ECM on cell signaling, the phosphorylation status of 46 kinases was examined. Increases in the phosphorylation of MAPK pathway-related proteins as well as sustained Akt phosphorylation were observed in BM-ECM cultures when compared to cultures grown on plasma-treated polystyrene. Blocking MEK1/2 or the PI3K pathway led to a significant reduction in LNCaP survival when cultured on BM-ECM in androgen-depleted conditions. The clinical relevance of these observations was determined by analyzing Erk phosphorylation in human bone metastatic prostate cancer versus non-metastatic prostate cancer, and increased phosphorylation was seen in the metastatic samples. Here we describe an engineered bone marrow model that mimics many features observed in patients and provides a platform for mechanistic in vitro studies.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
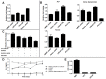
Figure 1. hMSC-LNCaP paracrine signaling.
Co-cultures of human mesenchymal stem cells (hMSCs) and prostate cancer cells permits paracrine signaling, induces osteogenesis, and supports prostate cancer survival. For panels A, B, C raw values were normalized to the negative control group. (A) hMSCs were treated with normal growth media, LNCaP conditioned media, PC3 conditioned media, or osteogenic media 14 days. They were then stained with alizarin red which was subsequently extracted and quantified to determine calcium deposition. (B) qRT-PCR for the specified transcripts. Each group was cultured as described in (A), and RNA subsequently extracted. (C) Indirect co-cultures of hMSCs with LNCaPs in androgen deleted media in the presence of the IGFR inhibitor AG1024, or IL6 inhibitors, AG490 and anti-IL6 mAb. Cells were plated in growth medium, allowed to recover for 24 h, and subsequently switched to androgen depleted medium plus inhibitor. LNCaP viability was measured after 7 days. (D) IL6 concentrations in conditioned culture medium. (E) IGF-1 concentrations in conditioned media after 48 hours from each cell type cultured alone. (*P<0.05; **P<0.01; ***P<0.001, n = 3, ± SEM).
Figure 2. Effect of bone marrow derived extracellular matrix (BM-ECM) on prostate cancer cells.
Bone marrow-derived extracellular matrix (BM-ECM) protects prostate cancer cells against androgen depletion and chemotherapy. For panels A, B, C raw values were normalized to the negative control group. (A) Growth of LNCaP cells over time cultured in androgen depleted media as measured via MTT. Equal numbers of LNCaPs were seeded to BM-ECM or PTP with an n of 4 per group per time point, and assayed on the appropriate day. (B) MDA-PCa-2bs were plated and switched to androgen deleted media as described in (A) and cell viability was determined after 4 days. MDA-PCa-2bs cells were also cultured on commercial ECM. (C) Cell viability of LNCaP cells after 72 hours in growth medium with 25 nM docetaxel. (*P<0.05; **P<0.01; ***P<0.001, n = 3, except as noted in (A), ± SEM).
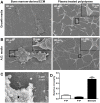
Figure 3. Scanning electron micrographs of LNCaP cells.
Bone marrow-derived extracellular matrix (BM-ECM) induces cluster like growth of LNCaPs that resists androgen depletion. LNCaPs were seeded to BM-ECM and PTP as detailed in Figure 2A. Scanning electron micrographs of 7 day cultures in (A) normal growth media and (B) androgen depleted media. (C) Higher magnification scanning electron micrograph of LNCaP (black asterisk) cells cultured on BM-ECM (white asterisk). (D) The circularity of LNCaPs on PTP in androgen depleted and growth medium and LNCaPs on BM-ECM in androgen depleted media was quantified from scanning electron microscopy images after 7 days in culture (*P<0.05; **P<0.01; ***P<0.001, ± SEM).
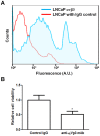
Figure 4. Integrin αvβ3 mediated adhesion to BM-ECM.
Expression of αvβ3 integrin on LNCaP cells mediates adhesion to bone marrow-derived extracellular matrix (BM-ECM). (A) Cell surface expression of αvβ3 integrin as measured by flow cytometry. (B) Anti-αvβ3 integrin antibody reduced cell adhesion to BM-ECM. Raw values were normalized to the control IgG group. (n = 3, *P<0.05; **P<0.01; ***P<0.001, ± SEM).
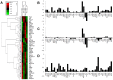
Figure 5. LNCaP phosphoproteomic analysis.
Differential phospho-proteomics of LNCaP cells cultured on bone marrow-derived extracellular matrix (BM-ECM). (A) Hierarchical clustering reveals LNCaPs on BM-ECM in androgen depleted media clustered most closely with LNCaPs cultured on PTP in growth media (B) Differential phosphorylation of LNCaPs on BM-ECM in androgen depleted media versus LNCaPs on PTP in androgen depleted media. (C) Differential phosphorylation of LNCaPs on PTP in androgen depleted media versus LNCaPs on PTP in growth media. (D) Differential phosphorylation of LNCaPs on BM-ECM in androgen depleted media versus LNCaPs on PTP in growth media.
Figure 6. Effect of inhibitors on LNCaP pathway activation and survival on BM-ECM.
Bone marrow-derived extracellular matrix (BM-ECM) induces differential signaling in LNCaP cells that mediates cell survival. Figure panels C, D, E raw values were normalized to the negative control group. (A) Western blot of LNCaPs indicates increased phospho-Erk1/2 and phospho-p90RSK in response to BM-ECM. (B) Effect of PI3K, LY294002, or MEK1/2, U0126, inhibitors on LNCaPs cultured on BM-ECM. Cells were plated at equal densities to BM-ECM or PTP in growth media and after 24 h switched to androgen depleted media ± inhibitors. Cell viability was measured after 7 days (n = 3). (C) Relative apoptosis as measured using a glucose 6-phosphate dehydrogenase assay after 72 hours. Clear bar indicates growth media, black bars androgen depleted media. Lysed cells are a positive control (n = 8). (D) LNCaPs were cultured on PTP indirectly with hMSCs, on BM-ECM, or in both conditions simultaneously while in androgen depleted media. Relative cell viability was measured after 7 days (n = 3, except as noted in (D), *P<0.05; **P<0.01; ***P<0.001, ± SEM). (E) Reductions in phospho-Erk1/2 in LNCaP cells on BM-ECM when treated with U0126 and LY294002.
Figure 7. Phospho-Erk immunohistochemistry in human prostate tissue sections.
Staining for Erk phosphorylation on a tissue microarray from primary tumor samples, and bone metastatic samples (A) Representative tissue sections stained for p-Erk from primary prostate tumor samples. Sample VI contained the only substantial positive staining in primary prostate samples. (B) Tissue sections stained for p-Erk from bone metastatic prostate cancer samples (scale bars are 200 µm).
Figure 8. Quantification of phospho-Erk immunohistochemistry.
The mean score plotted for each group taken from two blinded investigators (black circles). Staining in bone metastasis sections is significant over both normal tissue and primary tumor sections (n = 8 for normal prostate tissue, n = 72 for primary tumor, and n = 4 bone metastasis, ***P<0.001, ± std. dev.).
Similar articles
- Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling.
Luo J, Ok Lee S, Liang L, Huang CK, Li L, Wen S, Chang C. Luo J, et al. Oncogene. 2014 May 22;33(21):2768-78. doi: 10.1038/onc.2013.233. Epub 2013 Jun 24. Oncogene. 2014. PMID: 23792449 - Quantitative analysis of castration resistant prostate cancer progression through phosphoproteome signaling.
Lescarbeau RM, Kaplan DL. Lescarbeau RM, et al. BMC Cancer. 2014 May 8;14:325. doi: 10.1186/1471-2407-14-325. BMC Cancer. 2014. PMID: 24885093 Free PMC article. - Calcium phosphate scaffolds with defined interconnecting channel structure provide a mimetic 3D niche for bone marrow metastasized tumor cell growth.
Aveic S, Davtalab R, Vogt M, Weber M, Buttler P, Tonini GP, Fischer H. Aveic S, et al. Acta Biomater. 2019 Apr 1;88:527-539. doi: 10.1016/j.actbio.2019.02.030. Epub 2019 Feb 20. Acta Biomater. 2019. PMID: 30797105 - Endocrine/paracrine/autocrine survival factor activity of bone microenvironment participates in the development of androgen ablation and chemotherapy refractoriness of prostate cancer metastasis in skeleton.
Bogdanos J, Karamanolakis D, Tenta R, Tsintavis A, Milathianakis C, Mitsiades C, Koutsilieris M. Bogdanos J, et al. Endocr Relat Cancer. 2003 Jun;10(2):279-89. doi: 10.1677/erc.0.0100279. Endocr Relat Cancer. 2003. PMID: 12790789 Review. - Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications.
Mostaghel EA, Nelson PS. Mostaghel EA, et al. Best Pract Res Clin Endocrinol Metab. 2008 Apr;22(2):243-58. doi: 10.1016/j.beem.2008.01.003. Best Pract Res Clin Endocrinol Metab. 2008. PMID: 18471783 Free PMC article. Review.
Cited by
- Modelling bone metastasis in spheroids to study cancer progression and screen cisplatin efficacy.
Suurmond CE, Leeuwenburgh SCG, van den Beucken JJJP. Suurmond CE, et al. Cell Prolif. 2024 Sep;57(9):e13693. doi: 10.1111/cpr.13693. Epub 2024 Jun 20. Cell Prolif. 2024. PMID: 38899562 Free PMC article. - Functionalising silk hydrogels with hetero- and homotypic nanoparticles.
Kaewchuchuen J, Matthew SAL, Phuagkhaopong S, Bimbo LM, Seib FP. Kaewchuchuen J, et al. RSC Adv. 2024 Jan 22;14(5):3525-3535. doi: 10.1039/d3ra07634b. eCollection 2024 Jan 17. RSC Adv. 2024. PMID: 38259992 Free PMC article. - Impact of silk hydrogel secondary structure on hydrogel formation, silk leaching and in vitro response.
Egan G, Phuagkhaopong S, Matthew SAL, Connolly P, Seib FP. Egan G, et al. Sci Rep. 2022 Mar 8;12(1):3729. doi: 10.1038/s41598-022-07437-4. Sci Rep. 2022. PMID: 35260610 Free PMC article. - Advancing Treatment of Bone Metastases through Novel Translational Approaches Targeting the Bone Microenvironment.
Sethakorn N, Heninger E, Sánchez-de-Diego C, Ding AB, Yada RC, Kerr SC, Kosoff D, Beebe DJ, Lang JM. Sethakorn N, et al. Cancers (Basel). 2022 Feb 1;14(3):757. doi: 10.3390/cancers14030757. Cancers (Basel). 2022. PMID: 35159026 Free PMC article. Review. - Targeting Inflammatory Signaling in Prostate Cancer Castration Resistance.
Zhong S, Huang C, Chen Z, Chen Z, Luo JL. Zhong S, et al. J Clin Med. 2021 Oct 27;10(21):5000. doi: 10.3390/jcm10215000. J Clin Med. 2021. PMID: 34768524 Free PMC article. Review.
References
- Logothetis CJ, Lin SH (2005) Osteoblasts in prostate cancer metastasis to bone. Nat Rev Can 5: 21–28. - PubMed
- Blaszczyk N, Masri BA, Mawji NR, Ueda T, McAlinden G, et al. (2004) Osteoblast derived factors induce androgen-independent proliferation and expression of prostate-specific antigen in human prostate cancer cells. Clin Cancer Res 10: 1860–1869. - PubMed
- Vincent T, Mechti N (2005) Extracellular matrix in bone marrow can mediate drug resistance in myeloma. Leukemia Lymphoma 46: 803–811. - PubMed
- Andersen C, Bagi CM, Adams SW (2003) Intra-tibial injection of human prostate cancer cell line CWR22 elicits osteoblastic response in immunodeficient rats. J Musculoskel Neuron Interact 3: 148–155. - PubMed
- Moreau JE, Anderson K, Mauney JR, Nguygen T, Kaplan DL, et al. (2007) Tissue-Engineered Bone Serves as a Target for Metastasis of Human Breast Cancer in a Mouse Model. Cancer Res 67: 10304–10308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous