Ribosomal biogenesis genes play an essential and p53-independent role in zebrafish pancreas development - PubMed (original) (raw)
Ribosomal biogenesis genes play an essential and p53-independent role in zebrafish pancreas development
Elayne Provost et al. Development. 2012 Sep.
Abstract
Mutations in the human Shwachman-Bodian-Diamond syndrome (SBDS) gene cause defective ribosome assembly and are associated with exocrine pancreatic insufficiency, chronic neutropenia and skeletal defects. However, the mechanism underlying these phenotypes remains unclear. Here we show that knockdown of the zebrafish sbds ortholog fully recapitulates the spectrum of developmental abnormalities observed in the human syndrome, and further implicate impaired proliferation of ptf1a-expressing pancreatic progenitor cells as the basis for the observed pancreatic phenotype. It is thought that diseases of ribosome assembly share a p53-dependent mechanism. However, loss of p53 did not rescue the developmental defects associated with loss of zebrafish sbds. To clarify the molecular mechanisms underlying the observed organogenesis defects, we performed transcriptional profiling to identify candidate downstream mediators of the sbds phenotype. Among transcripts displaying differential expression, functional group analysis revealed marked enrichment of genes related to ribosome biogenesis, rRNA processing and translational initiation. Among these, ribosomal protein L3 (rpl3) and pescadillo (pes) were selected for additional analysis. Similar to knockdown of sbds, knockdown or mutation of either rpl3 or pes resulted in impaired expansion of pancreatic progenitor cells. The pancreatic phenotypes observed in rpl3- and pes-deficient embryos were also independent of p53. Together, these data suggest novel p53-independent roles for ribosomal biogenesis genes in zebrafish pancreas development.
Figures
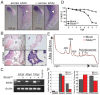
Fig. 1.
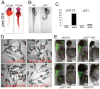
Zebrafish sbds is an essential developmental gene with high-level expression in pancreas and surrounding tissues. (A) Expression of sbds examined by in situ hybridization in wild-type zebrafish embryos at 120 hpf. The boxed pancreatic region is shown at higher magnification to illustrate pancreatic and peri-pancreatic expression (arrowheads) of sbds. (B) Adult zebrafish pancreas (boxed) expresses sbds. (C) SbdsSpl MO results in durable loss of sbds transcript as assessed by RT-PCR. (D) Survival curve for recipients of SbdsATG MO illustrates near complete embryonic lethality by 7 dpf, as compared with mock injected embryos. (E) Ribosome profile of lysate prepared from 48-hpf control and SbdsATG MO-injected embryos. (F) Quantification of ribosomal subunit ratios, monosomes and polysomes in control and SbdsATG MO-injected embryos. Error bars indicate mean ± s.e.m. i, intestine; L, liver; P, pancreas.
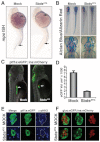
Fig. 2.
sbds knockdown in zebrafish embryos recapitulates human SDS organogenesis phenotypes. (A) At 24 hpf, loss of neutrophils in SbdsATG MO-injected embryos is revealed by in situ hybridization for the neutrophil marker _mpo. mpo_-positive cells were absent from both the anterior lateral mesoderm and the intermediate cell mass (top and bottom arrows, respectively). (B) At 120 hpf, abnormal cartilage of the gill arches and cerotohyal (ch) cartilage and bone (arrowheads) were observed in SbdsATG MO-injected embryos. (C) Visualization of _ptf1_-expressing pancreatic progenitor cells and differentiated beta cells in 72-hpf ptf1a:eGFP;ins:mCherry embryos (arrows). SbdsATG MO-injected embryos showed defects in _ptf1_-expressing pancreatic progenitors (green), whereas beta cells in the principal islet appeared normal (red). (D) Quantification of the volume of _ptf1a:eGFP_-expressing cells reveals an almost 75% reduction of pancreatic volume in SbdsATG MO-injected embryos. Error bars indicate s.e.m. (E) Immunofluorescence of phospho-histone H3 (pHH3, blue) marks proliferation in the exocrine pancreatic mass. Proliferation was decreased in the SbdsATG MO-injected embryos at 72 hpf. (F) High-resolution images of the pancreatic islet using gcga:eGFPia1;ins:mCherry double-transgenic zebrafish reveal normal islet architecture with peripheral alpha cells (green) and central beta cells (red) in SbdsATG MO-injected embryos at 72 hpf.
Fig. 3.
Loss of p53 does not rescue sbds organogenesis defects. (A) The body curvature observed in SbdsATG MO-injected zebrafish embryos is attenuated in SbdsATG/p53ATG MO-injected embryos at 48 hpf. (B) The p53 transcriptional targets Δ_113p53_ and p21 are induced upon injection of SbdsATG MO, as assessed by semi-quantitative PCR; co-injection of p53ATG MO abrogates this response. Error bars indicate s.e.m. (C) The 72-hpf pancreatic progenitor phenotype induced by the SbdsATG MO is not rescued by co-injection of p53ATG MO (arrows). (D) The absence of _mpo_-expressing neutrophils in the intermediate cell mass of SbdsATG/p53ATG MO-injected embryos was detected at 24 hpf (arrowheads). (E) Co-injection of p53Spl MO and SbdsATG MO into ptf1a:eGFP;ins:mCherry embryos does not rescue the defective expansion of pancreatic progenitor cells (arrows). (F) Genetic loss of p53 in p53zdf1/zdf1 embryos does not rescue the SbdsATG MO-induced neutrophil phenotype, as assessed by in situ hybridization for mpo at 24 hpf (arrowheads). (G) The SbdsATG MO-induced pancreatic progenitor defect is not rescued in p53zdf1/zdf1;ptf1a:eGFP;ins:mCherry embryos at 72 hpf.
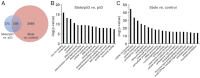
Fig. 4.
Gene set enrichment analysis of genes differentially expressed in SbdsATG MO-injected versus control and in SbdsATG/p53ATG MO-injected versus p53ATG MO-injected embryos. (A) Venn diagram depicting differentially expressed genes with a P<0.01 statistical cut-off. The red circle indicates genes differentially expressed in SbdsATG MO-injected versus control embryos, whereas the blue circle represents genes differentially expressed in SbdsATG/p53ATG MO-injected versus p53ATG MO-injected embryos. (B) The most highly enriched GO categories for genes upregulated in SbdsATG/p53ATG MO-injected versus p53ATG MO-injected embryos. (C) The most highly enriched GO categories for genes upregulated in SbdsATG MO-injected versus control embryos. For B and C, enriched GO categories are ranked based on the negative logarithm of their _P_-value. Note the high-level enrichment of genes related to ribosome biogenesis and rRNA processing in both comparisons.
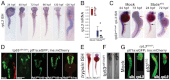
Fig. 5.
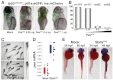
Knockdown of rpl3 phenocopies SbdsATG MO-induced defects in pancreas development. (A) Expression of rpl3 is widespread at 24 hpf and becomes progressively restricted to the endoderm, with high-level pancreatic expression at 120 hpf. The principal islet (I) is labeled by in situ hybridization for insulin (red). (B) rpl3 expression is decreased in SbdsATG MO-injected embryos, as quantified by microarray analysis of transcript abundance. Error bars indicate s.e.m. (C) In situ hybridization for rpl3 in SbdsATG MO-injected p53zdf1/zdf1 embryos confirms loss of rpl3 expression in somites (black arrows), central nervous system (white arrows), pharyngeal arches (red arrows) and intestine (yellow arrows) of developing embryos. (D) An MO dose-dependent defect in pancreas progenitor expansion was observed in Rpl3ATG MO-injected ptf1a:eGFP;ins:mCherry;p53zdf1/zdf1 embryos. (E) The small pancreas (arrow) of p53zdf1/zdf1 embryos injected with 0.5 ng Rpl3ATG MO is positive for trypsin expression by in situ hybridization. (F) p53zdf1/zdf1 embryos injected with 0.5 ng Rpl3ATG MO at 72 hpf exhibit small heads and hydroencephaly (yellow arrowhead). (G) rpl3hi2437/+;ptf1a:eGFP;ins:mCherry heterozygotes bearing a mutagenic rpl3 transgenic insertion were incrossed. rpl3hi2437/hi2437 homozygous embryos displayed disrupted pancreas progenitor expansion at 72 hpf. Injection of rpl3hi2437;ptf1a:eGFP;ins:mCherry with the p53ATG MO did not rescue the pancreatic progenitor phenotype of homozygous rpl3hi2437hi/2437 embryos. L, liver; P, pancreas; I, islet; Ex, exocrine pancreas.
Fig. 6.
The pes mutant phenocopies SbdsATG MO-induced p53-independent defects in pancreas development. (A) In situ hybridization at 24 hpf demonstrates pes expression in the eye and central nervous system. At 72 hpf, pes expression is detected in endoderm, including the developing pancreas. (B) The peshi2Tg/hi2Tg phenotype includes axis defects (tail curvature), a small dark head and a small eye. (C) Semi-quantitative RT-PCR of peshi2Tg/hi2Tg embryos reveals activation of the p53 target genes Δ_113p53_ (24-fold, P<0.0001) and p21 (2.4-fold, P<0.01). Error bars indicate s.e.m. (D) Loss of p53 does not rescue axis defects in peshi2Tg/hi2Tg embryos. Representative images of siblings (sib) generated either from a peshi2Tg/WT incross and injected with or without (Mock) p53ATG MO or p53Spl MO, or from a peshi2Tg/WT;p53zdf1/zdf1 incross. Asterisks indicate siblings displaying the pes phenotype. See
supplementary material
Fig. S6 for quantification. (E) peshi2Tg/hi2Tg embryos exhibit a p53-independent defect in expansion of _ptf1_-expressing pancreatic progenitor cells. The pancreatic progenitor defect in 72-hpf peshi2Tg/hi2Tg;ptf1a:eGFP;ins:mCherry embryos is not rescued by p53 MO-mediated knockdown, nor by genetic inactivation of p53 as assessed by incross of peshi2Tg/WT;p53zdf1/zdf1;ptf1a:eGFP;ins:mCherry fish.
Fig. 7.
MO knockdown of pes phenocopies SbdsATG MO-induced p53-independent defects in pancreas development. (A) Injection of PesATG MO into p53zdf1/zdf1 embryos results in a dose-dependent defect in pancreatic progenitor expansion, as assessed in 72-hpf ptf1a:eGFP;ins:mCherry embryos. (B) Quantification of three independent experiments scored for the percentage of embryos with wild-type pancreas following injection of PesATG MO into a p53zdf1/zdf1;ptf1a:eGFPjh1;ins:mCherryjh2 background. (C) The gross appearance of PesATG MO-injected embryos at 72 hpf includes a small head and eye but normal body axis. (D) Microarray quantification of pes transcript levels indicates an increase in pes expression in SbdsATG MO-injected p53zdf1/zdf1 embryos. (E) Increased abundance of pes transcripts in SbdsATG MO-injected p53zdf1/zdf1 embryos is confirmed by in situ hybridization. Error bars indicate s.e.m.
Similar articles
- Loss of Sbds in zebrafish leads to neutropenia and pancreas and liver atrophy.
Oyarbide U, Shah AN, Amaya-Mejia W, Snyderman M, Kell MJ, Allende DS, Calo E, Topczewski J, Corey SJ. Oyarbide U, et al. JCI Insight. 2020 Sep 3;5(17):e134309. doi: 10.1172/jci.insight.134309. JCI Insight. 2020. PMID: 32759502 Free PMC article. - In Vivo Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency.
Tourlakis ME, Zhang S, Ball HL, Gandhi R, Liu H, Zhong J, Yuan JS, Guidos CJ, Durie PR, Rommens JM. Tourlakis ME, et al. PLoS Genet. 2015 Jun 9;11(6):e1005288. doi: 10.1371/journal.pgen.1005288. eCollection 2015 Jun. PLoS Genet. 2015. PMID: 26057580 Free PMC article. - Defective ribosome assembly in Shwachman-Diamond syndrome.
Wong CC, Traynor D, Basse N, Kay RR, Warren AJ. Wong CC, et al. Blood. 2011 Oct 20;118(16):4305-12. doi: 10.1182/blood-2011-06-353938. Epub 2011 Jul 29. Blood. 2011. PMID: 21803848 - Shwachman-Diamond Syndrome: Molecular Mechanisms and Current Perspectives.
Bezzerri V, Cipolli M. Bezzerri V, et al. Mol Diagn Ther. 2019 Apr;23(2):281-290. doi: 10.1007/s40291-018-0368-2. Mol Diagn Ther. 2019. PMID: 30413969 Review. - Do ribosomopathies explain some cases of common variable immunodeficiency?
Khan S, Pereira J, Darbyshire PJ, Holding S, Doré PC, Sewell WA, Huissoon A. Khan S, et al. Clin Exp Immunol. 2011 Jan;163(1):96-103. doi: 10.1111/j.1365-2249.2010.04280.x. Epub 2010 Nov 9. Clin Exp Immunol. 2011. PMID: 21062271 Free PMC article. Review.
Cited by
- Haploinsufficiency for Core Exon Junction Complex Components Disrupts Embryonic Neurogenesis and Causes p53-Mediated Microcephaly.
Mao H, McMahon JJ, Tsai YH, Wang Z, Silver DL. Mao H, et al. PLoS Genet. 2016 Sep 12;12(9):e1006282. doi: 10.1371/journal.pgen.1006282. eCollection 2016 Sep. PLoS Genet. 2016. PMID: 27618312 Free PMC article. - Ribosomal proteins and human diseases: pathogenesis, molecular mechanisms, and therapeutic implications.
Wang W, Nag S, Zhang X, Wang MH, Wang H, Zhou J, Zhang R. Wang W, et al. Med Res Rev. 2015 Mar;35(2):225-85. doi: 10.1002/med.21327. Epub 2014 Aug 28. Med Res Rev. 2015. PMID: 25164622 Free PMC article. Review. - Loss of Sbds in zebrafish leads to neutropenia and pancreas and liver atrophy.
Oyarbide U, Shah AN, Amaya-Mejia W, Snyderman M, Kell MJ, Allende DS, Calo E, Topczewski J, Corey SJ. Oyarbide U, et al. JCI Insight. 2020 Sep 3;5(17):e134309. doi: 10.1172/jci.insight.134309. JCI Insight. 2020. PMID: 32759502 Free PMC article. - Loss of rps9 in Zebrafish Leads to _p53_-Dependent Anemia.
Chen C, Huang H, Yan R, Lin S, Qin W. Chen C, et al. G3 (Bethesda). 2019 Dec 3;9(12):4149-4157. doi: 10.1534/g3.119.400585. G3 (Bethesda). 2019. PMID: 31619461 Free PMC article. - Aldh1-expressing endocrine progenitor cells regulate secondary islet formation in larval zebrafish pancreas.
Matsuda H, Parsons MJ, Leach SD. Matsuda H, et al. PLoS One. 2013 Sep 17;8(9):e74350. doi: 10.1371/journal.pone.0074350. eCollection 2013. PLoS One. 2013. PMID: 24147152 Free PMC article.
References
- Allende M. L., Amsterdam A., Becker T., Kawakami K., Gaiano N., Hopkins N. (1996). Insertional mutagenesis in zebrafish identifies two novel genes, pescadillo and dead eye, essential for embryonic development. Genes Dev. 10, 3141–3155 - PubMed
- Boocock G. R., Morrison J. A., Popovic M., Richards N., Ellis L., Durie P. R., Rommens J. M. (2003). Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat. Genet. 33, 97–101 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK56211/DK/NIDDK NIH HHS/United States
- F32 DK077480/DK/NIDDK NIH HHS/United States
- DK080730/DK/NIDDK NIH HHS/United States
- DK61215/DK/NIDDK NIH HHS/United States
- R01 DK061215/DK/NIDDK NIH HHS/United States
- DK082060/DK/NIDDK NIH HHS/United States
- R41 DK082060/DK/NIDDK NIH HHS/United States
- DK077480/DK/NIDDK NIH HHS/United States
- T32 GM007814/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- RC4 DK090816/DK/NIDDK NIH HHS/United States
- R01 DK064646/DK/NIDDK NIH HHS/United States
- R25 GM059244/GM/NIGMS NIH HHS/United States
- R01 DK056211/DK/NIDDK NIH HHS/United States
- R01 DK080730/DK/NIDDK NIH HHS/United States
- 5R25GM059244-11/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous