KCa3.1 and TRPM7 channels at the uropod regulate migration of activated human T cells - PubMed (original) (raw)
KCa3.1 and TRPM7 channels at the uropod regulate migration of activated human T cells
Zerrin Kuras et al. PLoS One. 2012.
Abstract
The migration of T lymphocytes is an essential part of the adaptive immune response as T cells circulate around the body to carry out immune surveillance. During the migration process T cells polarize, forming a leading edge at the cell front and a uropod at the cell rear. Our interest was in studying the involvement of ion channels in the migration of activated human T lymphocytes as they modulate intracellular Ca(2+) levels. Ca(2+) is a key regulator of cellular motility. To this purpose, we created protein surfaces made of the bio-polymer PNMP and coated with ICAM-1, ligand of LFA-1. The LFA-1 and ICAM-1 interaction facilitates T cell movement from blood into tissues and it is critical in immune surveillance and inflammation. Activated human T lymphocytes polarized and migrated on ICAM-1 surfaces by random walk with a mean velocity of ∼6 µm/min. Confocal microscopy indicated that Kv1.3, CRAC, and TRPM4 channels positioned in the leading-edge, whereas KCa3.1 and TRPM7 channels accumulated in the uropod. The localization of KCa3.1 and TRPM7 at the uropod was associated with oscillations in intracellular Ca(2+) levels that we measured in this cell compartment. Further studies with blockers against Kv1.3 (ShK), KCa3.1 (TRAM-34), CRAC (SKF-96365), TRPM7 (2-APB), and TRPM4 (glibenclamide) indicated that blockade of KCa3.1 and TRPM7, and not Kv1.3, CRAC or TRPM4, inhibits the T cell migration. The involvement of TRPM7 in cell migration was confirmed with siRNAs against TRPM7. Downregulation of TRPM7 significantly reduced the number of migrating T cells and the mean velocity of the migrating T cells. These results indicate that KCa3.1 and TRPM7 selectively localize at the uropod of migrating T lymphocytes and are key components of the T cell migration machinery.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
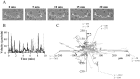
Figure 1. Fabrication of protein surface with intercellular adhesion molecule-1 (ICAM-1).
A. Structure of the terpolymer PNMP. B. Biotinylation of PNMP. The treatment of PNMP with succinic anhydride resulted in a carboxyl group which is then biotinylated by biotin-PEO-amine. The biotinylation allows a more specific protein binding compared to the binding of a carboxyl group. C. Diagram of protein patterning on PNMP. A glass coverslip is coated with a cationic layer of APTES and on top of this a layer of PNMP is deposited by spin-coating. The appropriate thickness of PNMP is achieved by UV irradiation and washing in pH 7.4 PBS. For the optimization of the thickness of the surfaces used in our experiments refer to the supplemental data shown in Table S1 which reports the experimental conditions necessary to achieve the appropriate PNMP thickness (200 nm). After these steps, streptavidin, which binds to the biotin, is added followed by addition of the biotinylated IgG and ICAM-1.
Figure 2. Movement of human activated CD3+ T cells on ICAM-1 surfaces by random walk.
A. Representative time-lapse bright-field microscopy of an activated T cell migrating on an ICAM-1 surface. Asterisks indicate the starting point of the cell of interest, and the arrow shows the direction of the movement. The dotted line represents the coming track whereas the continuous line is the covered distance. Scale bar = 5 µm. B. Time-dependent distribution of the migration velocity of two representative activated T cells each defined by either a continuous or dotted line. C. Tracks of 54 migrating T cells on ICAM-1 normalized to their starting coordinates. The x-y coordinates were determined in MetaMorph software. The final x–y values reached by 5 cells are out of the x/y axis ranges. The corresponding x–y coordinates are given for each cell at the end of the extended dotted lines.
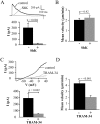
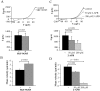
Figure 3. Selective role of KCa3.1 on the migration of activated CD3+ T cells.
A. ShK inhibition of native Kv1.3 currents. Representative Kv1.3 currents were recorded in activated T cells before (control) and after extracellular application of 10 nM ShK. Currents were elicited by depolarizing pulses to +50 mV from a holding potential (HP) of −80 mV every 30 s. The average inhibition of Kv1.3 currents is shown in the lower panel (n = 6). B. Mean velocity before and after ShK (n = 7). The effect of the Kv1.3 inhibitor ShK (10 nM) on cell migration was determined by following single cells for 20 min before and after treatment with the blocker. C. TRAM-34 inhibition of native KCa3.1 currents. Representative KCa3.1 currents were recorded in activated T cells before (control) and after extracellular application of 250 nM TRAM-34. Currents were induced from a HP of −80 mV by a ramp depolarization from −120 mV to +40 mV every 10 s. The average KCa3.1 currents in absence and presence of TRAM-34 are shown in the lower panel (n = 9). D. Mean velocity before and after TRAM-34 (n = 22). The effect of 250 nM TRAM-34 was determined by following single cells for 20 min before and after TRAM-34 application.
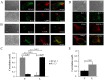
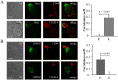
Figure 4. Differential localization of Kv1.3 and KCa3.1 in migrating T cells.
A. Distribution of Kv1.3 and KCa3.1 at the uropod. T cells were transiently transfected with either YFP-KCa3.1 or GFP-Kv1.3 (green) and stained with anti-CD44 antibody (uropod; red) without permeabilization. Yellow areas in the merge images indicate colocalization. Scale bar = 5 µm. B. Distribution of Kv1.3 and KCa3.1 at the leading-edge. T cells, transfected with either YFP-KCa3.1 or GFP-Kv1.3 (green) and stained with anti-CXCR-4 antibody (leading-edge; red) without permeabilization, were analyzed by confocal microscopy. Colocalization between the two proteins is indicated by yellow areas in the merge images. Scale bar = 5 µm. C. Correlation coefficients for KCa3.1 and Kv1.3 localization in the uropod (U) and leading-edge (L). The data are the average of n = 15 cells for KCa3.1 at the U and n = 8 at the L, and n = 16 for Kv1.3 at the U and n = 11 at the U from 2 healthy individuals. Statistical significance was established by one way ANOVA. D. Localization of native Kv1.3 in the leading-edge. T cells from one healthy individual were fixed and stained with extracellular anti-Kv1.3 antibody (green) together with antibodies either against CD44 (red; left) or CXCR-4 (red, right). Yellow colors in the merge images indicate strong correlation. Scale bar = 5 µm. E. Average Correlation coefficients of native Kv1.3 with the leading-edge (L) (n = 9) and the uropod (U) markers (n = 11).
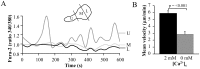
Figure 5. Intracellular Ca2+ concentrations and the effect of extracellular Ca2+ in migrating activated T cells.
A. Time-dependent intracellular Ca2+ levels in the uropod (U), mid-body (M) and leading-edge (L) in a representative migrating T cell. Cells were loaded with 1 µM Fura-2, and the Fura-2 340/380 ratio was quantified using the MetaMorph software. A scheme representing the different cell compartments of a polarized migrating cell is shown as inset. B. Effect of extracellular Ca2+ removal on T cell migration. The motility was determined by following the same cell in physiological [Ca2+]e (2 mM) and in Ca2+-free medium (0 Ca2+/EGTA). Control motility is the average motility over 20 min, while the motility in Ca2+ -free medium corresponds to the average of the last 5 min of 25 min in Ca2+-free medium. The data are the average of n = 17 cells from 2 different donors.
Figure 6. Differential distribution of Orai1 and TRPM7.
A. Confocal images of migrating activated T cells stained for Orai1 (green) together with either CD44 (red) or CXCR-4 (red). Yellow areas in the merge images indicate colocalization. Scale bar = 5 µm. The average correlation coefficients for the uropod (U, n = 21) and the leading-edge (L, n = 22) from 2 donors are shown in the right panel. B. Confocal images of migrating activated T cells that were fixed and stained for TRPM7 (green) together with either CD44 (red) or CXCR-4 (red). Correlation between two proteins is indicated by yellow areas in the merge images. Scale bar = 5 µm. The average correlation coefficients for the uropod (U, n = 29) and the leading-edge (L, n = 12) from 2 donors are shown in the right panel.
Figure 7. Selective inhibitory effect of TRPM7 blockade on activated T cell migration.
A. Lack of effect of SKF-96365 on TRPM7 currents. Representative TRPM7 currents in activated T cells were recorded before (control) and after extracellular application of 20 µM SKF-96365. Currents were induced from a HP of 0 mV by ramp depolarization from −100 mV to +100 mV every 10 s. The average current amplitude (lower panel) for TRPM7 channels was calculated at +100 mV (n = 8). B. Effect of SKF-96365 (20 µM) on T cell migration. T cell migration was measured in the same cell before and after treatment with the blocker. The data are the average of 18 cells from 2 individuals. C. Inhibition of native TRPM7 currents by 2-APB. Representative TRPM7 currents in activated T cells were recorded before (control) and after increasing concentrations of 2-APB. Currents were elicited like in A. The corresponding average current amplitudes are shown in the bottom panel (n = 7). D. Effect of 2-APB on T cell migration. T cell migration was measured in the same cell before and after treatment with progressive concentrations of 2-APB. The data are the mean of 15 cells from 2 individuals. Statistical significance in panels C and D was determined by One Way Repeated Measures ANOVA and post-hoc testing for significance was determined by Holm-Sidak method. * represents statistical significance in the treatment groups as compared to the control.
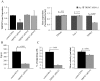
Figure 8. TRPM7 channels are crucial for T cell migration.
A. Left Panel: TRPM7 gene expression is reduced by siRNA1. TRPM7 gene expression was quantified by RT-qPCR and is given in fold change relative to GAPDH expression. The data are normalized to control RNA transfected cells and correspond to mean ± SEM of 3 healthy donors each in quadruplicate. Statistical significance was determined by One Way ANOVA, while post-hoc testing was done by Holm-Sidak method. * indicates statistical significance. Right Panel: Specificity of TRPM7 siRNA. Activated T cells were transfected with TRPM7 siRNA1 for 48 hours and the gene expression for TRPM4, Orai1 and TRPM7 was quantified by RT-qPCR. The data are shown as fold change in gene expression relative to GAPDH and are normalized to control RNA transfected cells. Data are presented as mean ± SEM for 3 independent donors, with samples in quadruplicate. B. TRPM7 knockdown decreases TRPM7 currents. The average TRPM7 current was significantly decreased in TRPM7 siRNA1 transfected cells (n = 13) compared to cells transected with control RNA (n = 10). Transected cells were visualized by GFP expression. C. Effect of TRPM7 siRNA1 on T cell migration. On the left panel is reported the % of cells migrating. The total number of cells were for control RNA 626 (n = 5 experiments; 2 donors) and for TRPM7 siRNA1 796 (n = 8 experiments; 2 donors). Right panel: T cell migration was measured in TRPM7 siRNA1 transfected cells (n = 40) and compared to control RNA transfected cells (n = 49) from 2 individuals.
Similar articles
- Selective inhibition of KCa3.1 channels mediates adenosine regulation of the motility of human T cells.
Chimote AA, Hajdu P, Kucher V, Boiko N, Kuras Z, Szilagyi O, Yun YH, Conforti L. Chimote AA, et al. J Immunol. 2013 Dec 15;191(12):6273-80. doi: 10.4049/jimmunol.1300702. Epub 2013 Nov 13. J Immunol. 2013. PMID: 24227782 Free PMC article. - Selective activation of KCa3.1 and CRAC channels by P2Y2 receptors promotes Ca(2+) signaling, store refilling and migration of rat microglial cells.
Ferreira R, Schlichter LC. Ferreira R, et al. PLoS One. 2013 Apr 19;8(4):e62345. doi: 10.1371/journal.pone.0062345. Print 2013. PLoS One. 2013. PMID: 23620825 Free PMC article. - The Ca(2+)-activated K(+) channel KCa3.1 compartmentalizes in the immunological synapse of human T lymphocytes.
Nicolaou SA, Neumeier L, Peng Y, Devor DC, Conforti L. Nicolaou SA, et al. Am J Physiol Cell Physiol. 2007 Apr;292(4):C1431-9. doi: 10.1152/ajpcell.00376.2006. Epub 2006 Dec 6. Am J Physiol Cell Physiol. 2007. PMID: 17151145 Free PMC article. - Role of KCa3.1 Channels in Modulating Ca2+ Oscillations during Glioblastoma Cell Migration and Invasion.
Catacuzzeno L, Franciolini F. Catacuzzeno L, et al. Int J Mol Sci. 2018 Sep 29;19(10):2970. doi: 10.3390/ijms19102970. Int J Mol Sci. 2018. PMID: 30274242 Free PMC article. Review.
Cited by
- The role of KCa3.1 channels in cardiac fibrosis induced by pressure overload in rats.
Zhao LM, Wang LP, Wang HF, Ma XZ, Zhou DX, Deng XL. Zhao LM, et al. Pflugers Arch. 2015 Nov;467(11):2275-85. doi: 10.1007/s00424-015-1694-4. Epub 2015 Feb 27. Pflugers Arch. 2015. PMID: 25715999 - Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke?
Leng T, Shi Y, Xiong ZG, Sun D. Leng T, et al. Prog Neurobiol. 2014 Apr;115:189-209. doi: 10.1016/j.pneurobio.2013.12.008. Epub 2014 Jan 24. Prog Neurobiol. 2014. PMID: 24467911 Free PMC article. Review. - Functionalized liposomes loaded with siRNAs targeting ion channels in effector memory T cells as a potential therapy for autoimmunity.
Hajdu P, Chimote AA, Thompson TH, Koo Y, Yun Y, Conforti L. Hajdu P, et al. Biomaterials. 2013 Dec;34(38):10249-57. doi: 10.1016/j.biomaterials.2013.09.019. Epub 2013 Sep 27. Biomaterials. 2013. PMID: 24075407 Free PMC article. - A Compartmentalized Reduction in Membrane-Proximal Calmodulin Reduces the Immune Surveillance Capabilities of CD8+ T Cells in Head and Neck Cancer.
Chimote AA, Gawali VS, Newton HS, Wise-Draper TM, Conforti L. Chimote AA, et al. Front Pharmacol. 2020 Feb 28;11:143. doi: 10.3389/fphar.2020.00143. eCollection 2020. Front Pharmacol. 2020. PMID: 32184726 Free PMC article. - The inhibitory effect of adenosine on tumor adaptive immunity and intervention strategies.
Wang L, Zhang J, Zhang W, Zheng M, Guo H, Pan X, Li W, Yang B, Ding L. Wang L, et al. Acta Pharm Sin B. 2024 May;14(5):1951-1964. doi: 10.1016/j.apsb.2023.12.004. Epub 2023 Dec 16. Acta Pharm Sin B. 2024. PMID: 38799637 Free PMC article. Review.
References
- von Andrian UH, Mackay CR (2000) T-Cell Function and Migration: Two Sides of the Same Coin. New England Journal of Medicine 343: 1020–1034. - PubMed
- Friedl P, Weigelin B (2008) Interstitial leukocyte migration and immune function. Nat Immunol 9: 960–969. - PubMed
- Gomez TS, Billadeau DD (2008) T Cell Activation and the Cytoskeleton: You Can’t Have One Without the Other. Advances in Immunology. Volume 97 ed: Academic Press. 1–64. - PubMed
- Schwab A, Hanley P, Fabian A, Stock C (2008) Potassium Channels Keep Mobile Cells on the Go. Physiology 23: 212–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous