Integration of β-catenin, sirtuin, and FOXO signaling protects from mutant huntingtin toxicity - PubMed (original) (raw)
Integration of β-catenin, sirtuin, and FOXO signaling protects from mutant huntingtin toxicity
J Alex Parker et al. J Neurosci. 2012.
Abstract
One of the current challenges of neurodegenerative disease research is to determine whether signaling pathways that are essential to cellular homeostasis might contribute to neuronal survival and modulate the pathogenic process in human disease. In Caenorhabditis elegans, sir-2.1/SIRT1 overexpression protects neurons from the early phases of expanded polyglutamine (polyQ) toxicity, and this protection requires the longevity-promoting factor daf-16/FOXO. Here, we show that this neuroprotective effect also requires the DAF-16/FOXO partner bar-1/β-catenin and putative DAF-16-regulated gene ucp-4, the sole mitochondrial uncoupling protein (UCP) in nematodes. These results fit with a previously proposed mechanism in which the β-catenin FOXO and SIRT1 proteins may together regulate gene expression and cell survival. Knockdown of β-catenin enhanced the vulnerability to cell death of mutant-huntingtin striatal cells derived from the HdhQ111 knock-in mice. In addition, this effect was compensated by SIRT1 overexpression and accompanied by the modulation of neuronal UCP expression levels, further highlighting a cross-talk between β-catenin and SIRT1 in the modulation of mutant polyQ cytoxicity. Taken together, these results suggest that integration of β-catenin, sirtuin and FOXO signaling protects from the early phases of mutant huntingtin toxicity.
Figures
Figure 1.
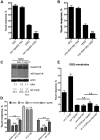
β-Catenin and ucp-4 are required for neuroprotection by sir-2.1 in 128Q nematodes. A, bar-1/β-catenin null mutation enhanced touch insensitivity at the tail of 128Q nematodes. No change was detected in 19Q animals. ***p < 0.001 compared to 128Q alone. B**, ucp-4 deletion enhanced 128Q neuronal dysfunction. No change was detected in 19Q animals. ***p < 0.001 compared to 128Q alone. **_C_**, 128Q transgene expression is unchanged at the protein and mRNA levels in 128Q nematodes bearing _bar-1_ or _ucp-4_ LOF. **_D_**, Aggravation of neuron dysfunction by _sir-2.1_ LOF (_sir-2.1_(_ok434_)) was suppressed in animals specifically overexpressing (O/E) wild-type SIR-2.1 in touch receptor neurons under the control of the promoter of the _mec-3_ gene with no effect detected in animals overexpressing empty vector (ID1296 and ID1297; see Table 1). Aggravation of neuron dysfunction by _bar-1_ LOF (_bar-1_(_ga80_)) was suppressed in animals specifically overexpressing wild-type BAR-1 in touch receptor neurons under the control of the promoter of the _mec-3_ gene, with no effect detected in detected in animals overexpressing empty vector (ID1316 and ID1317; see Table 1). Shown are data compiled from two independent extrachromosomal arrays per genotype. ***_p_ < 0.001 versus empty vector. n.s., Not significant. **_E_**, Neuroprotection by increased Sir2 dosage (_sir-2.1_(_O/E_)) against 128Q toxicity was lost in animals mutant for _bar-1_ or _ucp-4_. *_p_ < 0.001 versus 128Q; **_p_ < 0.001 versus _sir-2.1_(_O/E_) alone. Data in **_A_**, **_B_**, and **_E_** are means ± SEM with >200 animals per genotype (50–60 animals per independent experiment for a total of at least 4 independent experiments). Data for touch tests in strains expressing extrachromosomal arrays (D**) are means ± SEM with >60 animals per array (20–30 animals per independent experiment for a total of at least 3 independent experiments).
Figure 2.
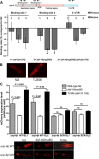
The UCP-4 promoter is regulated by DAF-16. A, DAF-16 binds to the UCP-4 promoter. The 5′ region of UCP-4 has three consensus DAF-16 binding sites (top; only 2 sites are shown; binding site 1 has two closely located consensus sites) that are separated by 3.7 kb. One of the binding sites is located 894 bp upstream of the ATG (binding site 2), while two others are located 4.655 and 4.964 kb upstream of ATG. Since the latter sites are closely situated, primers were designed for the site at 4.655 kb (binding site 1). Primers were also designed for region 654 bp downstream of the stop codon as a control site in the 3′-region. Chromatin immunoprecipitation (bottom) using anti-DAF-16 antibody (bottom) using N2, daf-2(e1370), daf-16(mgDf50), and daf-16(mgDf50);daf-2(e1370). The binding was normalized to that of N2, and effects for which p < 0.01 were considered significant. *_p_ < 0.005 compared to N2. **_B_**, Representative images for DAF-16 overexpression to increase the activity of the UCP-4 promoter (1768 bp) in late L4 _C. elegans_ nematodes as inferred from quantifying the intensity of _mCherry_ signals in the pharynx (see Results). **_C_**, The effects of DAF-16 on the activity of the _ucp-4_ promoter requires binding site 2. Constructs encoding _mCherry_ under the control of the _ucp-4_ promoter (1768 bp), which carried a wild-type (_ucp-4p_ WT) or scrambled (_ucp-4p_ SCR) binding site 2, were stably expressed in N2, _daf-16_(_mu86_) or TJ356 strain, and _mCherry_ signals were quantified as indicated in **_B_**. Data are mean ± SEM as compiled from two independent arrays (SL1, SL2) per genotype, and >60 animals per array (20–30 animals per independent experiment for a total of at least 3 independent experiments).
Figure 3.
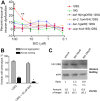
The GSK-3β inhibitor BIO is neuroprotective via FOXO signaling in 128Q nematodes. A, Neuron dysfunction in 128Q nematodes is higher compared to 19Q nematodes, with about 85% of the 128Q animals having a defective response to touch (Parker et al., 2005). BIO strongly rescues expanded polyQ neurotoxicity at 100–33.3 μ
m
(*p < 0.001 and **_p_ < 0.01 versus DMSO controls) with no effect in 19Q animals. BIO rescuing activity was lost in mutants for _daf-16_, _sir-2.1_, _bar-1_, and _ucp-4_. Dilution factor is 3×. Percent rescue was calculated from percentages of touch response as ((test − control)/(100 − control) * 100). Data are means ± SEM with >200 animals per point for all genotypes (50–60 animals per independent experiment for a total of at least 4 independent experiments). The percentages of touch response (means ± SEM) were 52 ± 3% in 19Q animals treated with vehicle, 23 ± 2% in 128Q animals treated with vehicle, 46 ± 3% in 128Q animals treated with 100 μ
m
BIO, and 34.6 ± 4% in 128Q animals treated with 33.3 μ
m
BIO. B, BIO reduced axonal swelling in PLM cells of 128Q animals (*p < 0.002 versus DMSO controls). Data are means ± SEM with >100 animals per treatment (25–30 animals per independent experiment for a total of at least 4 independent experiments). C, BIO treatment (50 μ
m
, 100 μ
m
) does not modify transgene expression in 128Q nematodes. n.s., Not significant.
Figure 4.
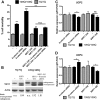
β-Catenin and UCP2/UCP4 modulate the survival of mutant htt striatal cells from HdhQ111 mice. In these assays, cells were subjected to serum deprivation. A, Representative graph showing that 109Q/109Q cells were more susceptible to cell death compared to 7Q/7Q cells (N = 3 with SD; *p < 0.01 compared to 7Q/7Q). B, Effects of reducing htt levels. Htt siRNA reduced 109Q/109Q cell mortality (N = 3 with SD; **p < 0.001 versus untreated). Scramble RNA showed no effect. ns, Not significant. C, Representative Western blot showing that Htt siRNA treatment reduces Htt expression levels in 7Q/7Q and 109Q/109Q striatal cells. In the following panels, data are normalized due to the variability of striatal cell survival after serum deprivation across experiments. D, Effects of reducing β-catenin levels and of BIO (0.5 μ
m
) treatment. Left, β-Catenin siRNA enhanced 109Q/109Q cell mortality (N = 3 with SD; *p < 0.01 versus untreated). Scramble RNA showed no effect. BIO treatment reduced cell mortality (N = 3 with SD; **p < 0.05 versus DMSO controls). When β-catenin siRNA and BIO were combined, no change in cell mortality was detected compared to cells treated with scramble RNA and DMSO (N = 3 with SD). Right, β-catenin siRNA and BIO showed no effect in 7Q/7Q cells. E, Mutant htt expression was unchanged by treatment with BIO or β-catenin siRNA. β-Catenin levels are increased by BIO (N = 3, p < 0.02) and reduced by β-catenin siRNA (n = 3, p < 0.05). Scramble RNA had no effect. E, Effects of reducing SIRT1, UCP2, and UCP4 (N = 4 with SD). UCP2 siRNA enhanced 109Q/109Q cell mortality (*p < 0.01 versus untreated) with no effect detected in 7Q/7Q cells. UCP4 siRNA reduced 109Q/109Q cell mortality (*p < 0.01 versus untreated) with no effect detected in 7Q/7Q cells. Scramble RNAs had no effect. G, Mutant htt expression was unchanged by siRNAs against SIRT1, UCP2, and UCP4. Shown are representative Western blots. Scramble RNAs showed no effect. H, At left is a representative Western blot image showing that 109Q/109Q cells have lower SIRT1 levels upon SIRT1 siRNA treatment (N = 3; p < 0.05). Quantitative RT-PCR experiments indicated that UCP2 and UCP4 siRNA decreased mRNA levels of UCP2 and UCP4, respectively (N = 5 with SD; p < 0.05). The effect of UCP2/4 siRNAs on target protein expression could not be evaluated, as the antisera were repeatedly unable to detect any protein in Western blot experiments, and scramble RNAs showed no effect. For all panels, N indicates the number of independent experiments performed.
Figure 5.
Effects of β-catenin and SIRT1 in mutant htt striatal cells from HdhQ111 mice. A, Mutant htt cells showed increased susceptibility to cell death induced by serum deprivation (***p < 0.001 compared to 7Q/7Q). SIRT1 overexpression slightly enhanced the survival of 109Q/109Q striatal cells with no effects in 7Q/7Q cells (**p < 0.01 compared to untreated 109Q/109Q). Reducing β-catenin enhanced the mortality of 109Q/109Q striatal cells with no effects in 7Q/7Q cells (***p < 0.001 compared to untreated 109Q/109Q). When SIRT1 overexpression was combined with β-catenin reduction in 109Q/109Q striatal cells, the detrimental effect of reducing β-catenin was compensated by SIRT1 overexpression (N (number of experiments performed) = 3 with SD; ***p < 0.001 compared to β-catenin reduction). n.s., Not significant. B, Representative Western blots showing that striatal cells transfected with the SIRT1 construct (an active variant lacking an internal segment in the N terminus) have increased SIRT1 levels. n.a., Not applicable. C, Effects of reducing β-catenin or overexpressing SIRT1 on the expression levels of UCP2. The expression of the UCP4 gene is downregulated by β-catenin siRNA with no effect by SIRT1 overexpression (O/E) in 109Q/109Q cells (N = 4 with SD; *p < 0.05). No effect was detected in 7Q/7Q cells. ns, Not significant. Scramble RNA (β-catenin) showed no effect on the UCP2/UCP4 gene expression levels (C, D).
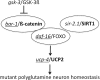
Figure 6.
Working model for the role of bar-1/β-catenin, daf-16/FOXO and sir-2.1/SIRT1 in the regulation of mutant polyglutamine neuron homeostasis. The effects observed in C. elegans and mouse striatal cells, two models of the early cytotoxicity/cell-vulnerability induced by mutant htt, are summarized in the context of prior knowledge on the binding of BAR-1/β-catenin to DAF-16/FOXO (Essers et al., 2005) and the role of GSK-3/β-catenin and SIRT1 in the modulation of FOXO activity in the nucleus and its ability to regulate gene expression (Greer and Brunet, 2008; Landis and Murphy, 2010; Yen et al., 2011). Genes required for neuroprotection by sir-2.1 in C. elegans as previously reported (Parker et al., 2005) or indicated herein are underlined. Bold indicates gene activity in mouse striatal cells as suggested by the ability of SIRT1 overexpression to compensate for the detrimental effect of reducing β-catenin on cell survival, and by the effects on UCP2 expression levels.
Similar articles
- Cross-talk between canonical Wnt signaling and the sirtuin-FoxO longevity pathway to protect against muscular pathology induced by mutant PABPN1 expression in C. elegans.
Pasco MY, Catoire H, Parker JA, Brais B, Rouleau GA, Néri C. Pasco MY, et al. Neurobiol Dis. 2010 Jun;38(3):425-33. doi: 10.1016/j.nbd.2010.03.002. Epub 2010 Mar 19. Neurobiol Dis. 2010. PMID: 20227501 - The evolutionarily conserved longevity determinants HCF-1 and SIR-2.1/SIRT1 collaborate to regulate DAF-16/FOXO.
Rizki G, Iwata TN, Li J, Riedel CG, Picard CL, Jan M, Murphy CT, Lee SS. Rizki G, et al. PLoS Genet. 2011 Sep;7(9):e1002235. doi: 10.1371/journal.pgen.1002235. Epub 2011 Sep 1. PLoS Genet. 2011. PMID: 21909281 Free PMC article. - Functional interaction between beta-catenin and FOXO in oxidative stress signaling.
Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Essers MA, et al. Science. 2005 May 20;308(5725):1181-4. doi: 10.1126/science.1109083. Science. 2005. PMID: 15905404 - Integration of diverse inputs in the regulation of Caenorhabditis elegans DAF-16/FOXO.
Landis JN, Murphy CT. Landis JN, et al. Dev Dyn. 2010 May;239(5):1405-12. doi: 10.1002/dvdy.22244. Dev Dyn. 2010. PMID: 20140911 Free PMC article. Review. - The interaction between FOXO and SIRT1: tipping the balance towards survival.
Giannakou ME, Partridge L. Giannakou ME, et al. Trends Cell Biol. 2004 Aug;14(8):408-12. doi: 10.1016/j.tcb.2004.07.006. Trends Cell Biol. 2004. PMID: 15308206 Review.
Cited by
- Genetic Screen in Adult Drosophila Reveals That dCBP Depletion in Glial Cells Mitigates Huntington Disease Pathology through a Foxo-Dependent Pathway.
Martin E, Heidari R, Monnier V, Tricoire H. Martin E, et al. Int J Mol Sci. 2021 Apr 9;22(8):3884. doi: 10.3390/ijms22083884. Int J Mol Sci. 2021. PMID: 33918672 Free PMC article. - High Levels of SIRT1 Expression as a Protective Mechanism Against Disease-Related Conditions.
Elibol B, Kilic U. Elibol B, et al. Front Endocrinol (Lausanne). 2018 Oct 15;9:614. doi: 10.3389/fendo.2018.00614. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30374331 Free PMC article. Review. - The brain, sirtuins, and ageing.
Satoh A, Imai SI, Guarente L. Satoh A, et al. Nat Rev Neurosci. 2017 May 18;18(6):362-374. doi: 10.1038/nrn.2017.42. Nat Rev Neurosci. 2017. PMID: 28515492 Review. - Dietary phytochemicals and neuro-inflammaging: from mechanistic insights to translational challenges.
Davinelli S, Maes M, Corbi G, Zarrelli A, Willcox DC, Scapagnini G. Davinelli S, et al. Immun Ageing. 2016 Apr 14;13:16. doi: 10.1186/s12979-016-0070-3. eCollection 2016. Immun Ageing. 2016. PMID: 27081392 Free PMC article. Review. - The neurodegenerative effects of selenium are inhibited by FOXO and PINK1/PTEN regulation of insulin/insulin-like growth factor signaling in Caenorhabditis elegans.
Estevez AO, Morgan KL, Szewczyk NJ, Gems D, Estevez M. Estevez AO, et al. Neurotoxicology. 2014 Mar;41(100):28-43. doi: 10.1016/j.neuro.2013.12.012. Epub 2014 Jan 6. Neurotoxicology. 2014. PMID: 24406377 Free PMC article.
References
- Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005b;6:829–840. - PubMed
- Anselmi CV, Malovini A, Roncarati R, Novelli V, Villa F, Condorelli G, Bellazzi R, Puca AA. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12:95–104. - PubMed
- Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell. 2006;125:1165–1177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous