Endogenous TDP-43, but not FUS, contributes to stress granule assembly via G3BP - PubMed (original) (raw)
Endogenous TDP-43, but not FUS, contributes to stress granule assembly via G3BP
Anaïs Aulas et al. Mol Neurodegener. 2012.
Erratum in
- Erratum to: Endogenous TDP-43, but not FUS, contributes to stress granule assembly via G3BP.
Aulas A, Stabile S, Vande Velde C. Aulas A, et al. Mol Neurodegener. 2015 Sep 3;10:45. doi: 10.1186/s13024-015-0041-8. Mol Neurodegener. 2015. PMID: 26337162 Free PMC article. No abstract available.
Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the selective loss of upper and lower motor neurons, a cell type that is intrinsically more vulnerable than other cell types to exogenous stress. The interplay between genetic susceptibility and environmental exposures to toxins has long been thought to be relevant to ALS. One cellular mechanism to overcome stress is the formation of small dense cytoplasmic domains called stress granules (SG) which contain translationally arrested mRNAs. TDP-43 (encoded by TARDBP) is an ALS-causative gene that we have previously implicated in the regulation of the core stress granule proteins G3BP and TIA-1. TIA-1 and G3BP localize to SG under nearly all stress conditions and are considered essential to SG formation. Here, we report that TDP-43 is required for proper SG dynamics, especially SG assembly as marked by the secondary aggregation of TIA-1. We also show that SG assembly, but not initiation, requires G3BP. Furthermore, G3BP can rescue defective SG assembly in cells depleted of endogenous TDP-43. We also demonstrate that endogenous TDP-43 and FUS do not have overlapping functions in this cellular process as SG initiation and assembly occur normally in the absence of FUS. Lastly, we observe that SG assembly is a contributing factor in the survival of neuronal-like cells responding to acute oxidative stress. These data raise the possibility that disruptions of normal stress granule dynamics by loss of nuclear TDP-43 function may contribute to neuronal vulnerability in ALS.
Figures
Figure 1
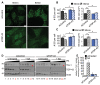
TIA-1 secondary aggregation is impaired by TDP-43 depletion. (A-D) Cells where transfected with control or TDP-43 siRNA for 72 hr and subsequently treated with SA. (A) Representative images of SG in HeLa cells at 60 and 90 min. Scale bar, 10 μm. (B & C) Quantification of SG number and size by semi-automatic analysis using ImageJ at 60 and 90 min after SA treatment in (B) HeLa and (C) SK-N-SH cells. The means of 3 independent experiments ± SEM are plotted. * p<0.05. (D) siRNA transfected HeLa cells were treated with or without SA and collected 1 hr post-SA. Cytoplasmic extracts were digested with 0, 0.1, 0.2, 0.4, 0.8 or 1.6 mg/ml Proteinase K and assayed by immunoblot. TIA-1 is more protease-sensitive when TDP-43 is absent (arrows). Data is representative of 3 independent experiments. (E) Quantification of remaining TIA-1 protein following treatment with 0. 8 mg/ml Proteinase K, expressed relative to untreated samples.
Figure 2
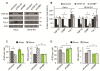
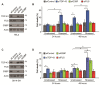
The TDP-43 target gene G3BP is required for SG assembly. (A) Western blot to confirm efficacy of indicated siRNAs in HeLa (left panel) and in SK-N-SH (right panel) cells. Actin serves as a loading control. (B) Quantification of protein levels demonstrating that G3BP does not regulate TDP-43 in HeLa (left panel) nor in SK-N-SH (right panel). (C & D) Quantification of individual SG size and number in siRNA treated cells, at 60 and 90 min after SA treatment in (C) HeLa cells and (D) SK-N-SH cells. The means of 3 independent experiments ± SEM are plotted. * p < 0.05. siControl in Figure 2C is the same presented in Figure 4A,B. siControl in Figure 2D is the same presented in Figure 1C.
Figure 3
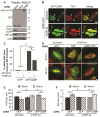
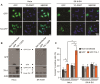
Blocked SG assembly in TDP-43 depleted cells is rescued by G3BP-GFP. (A - F) SG formation and resolution were assessed in HeLa cells transfected with control or TDP- 43 siRNA for 48 hr, transfected with GFP or G3BP-GFP for 24 hr and subsequently treated with SA. Cover slips were collected before SA treatment (A – C) and at 60 and 90 min after stress (D - F). (A) Western blot confirming expression of transfected plasmids. Actin serves as a loading control. (B) Representative micrographs of quantified cells with spontaneous SG formation. Asterisks and arrows indicate cells expressing G3BP-GFP with or without spontaneously forming SG, respectively. Scale bar, 20 μm. (C) Quantification of cells with spontaneous SG formation (SG formation in the absence of stress). Cells were scored as SG positive when they had at least two TIA-1 foci of a minimal size of 0.75 μm2. (D) Representative images quantified for SG number and size by semi-automatic analysis using ImageJ. Scale bar, 10 μm. (E & F) Number and size of SG were quantified at 60 and 90 min after SA treatment by semi-automatic analysis using ImageJ. The means of 3 independent experiments ± SEM are plotted. * p < 0.05.
Figure 4
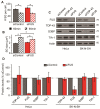
Endogenous FUS does not function in SG assembly in response to sodium arsenite. (A - E) SG were assessed in HeLa cells transfected with control or FUS siRNA for 72 hr and subsequently treated with SA. (A & B) Quantification of individual SG size and number of SG per cell. (C & D) Western blots and quantification of protein levels demonstrating that FUS does not regulate G3BP, TIA-1 or TDP-43, in HeLa cells (left panel) nor in SK-N-SH cells (right panel). Actin serves as a loading control. The means of 3 independent experiments ± SEM are plotted. * p < 0.05
Figure 5
Neuronal-like cells have increased vulnerability to blocked SG assembly. (A & B) HeLa and (C & D) SK-N-SH cells treated with the indicated siRNAs for 48 hr and then exposed to SA for 30 min. (A & C) Western blots confirming siRNA transfections in (A) HeLa and (D) SK-N-SH cells. Actin serves as a loading control. (B & D) Cell death determined by trypan blue exclusion at the indicated times following SA treatment in (B) HeLa and (D) SK-N-SH cells. The mean percentage of cell death is presented ± SEM of 3 independents experiments. * p < 0.05
Figure 6
HeLa cells have increased cell sensitivity to sodium arsenite in the presence of modestly elevated GFP-TIA-1 expression. (A–C) HeLa (left panels) and SK-N-SH (right panels) cells were transfected with GFP or GFP-TIA-1 for 48 hr. (A) Representative images of HeLa (left panels) and SK-N-SH (right panels) cells transfected with GFP or GFP-TIA-1 for 48 hr and then stained with TO-PRO3 to label nuclei. (B) Western blot confirming TIA-1 expression. Asterisk (*) indicates non-specific bands from TIA-1 antibody. (C) Cell viability was assessed by Annexin V labelling in HeLa and SK-N-SH. The means of 3 independent experiments ± SEM are plotted. * p<0.05
Similar articles
- Small-Molecule Modulation of TDP-43 Recruitment to Stress Granules Prevents Persistent TDP-43 Accumulation in ALS/FTD.
Fang MY, Markmiller S, Vu AQ, Javaherian A, Dowdle WE, Jolivet P, Bushway PJ, Castello NA, Baral A, Chan MY, Linsley JW, Linsley D, Mercola M, Finkbeiner S, Lecuyer E, Lewcock JW, Yeo GW. Fang MY, et al. Neuron. 2019 Sep 4;103(5):802-819.e11. doi: 10.1016/j.neuron.2019.05.048. Epub 2019 Jul 1. Neuron. 2019. PMID: 31272829 Free PMC article. - TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1.
McDonald KK, Aulas A, Destroismaisons L, Pickles S, Beleac E, Camu W, Rouleau GA, Vande Velde C. McDonald KK, et al. Hum Mol Genet. 2011 Apr 1;20(7):1400-10. doi: 10.1093/hmg/ddr021. Epub 2011 Jan 21. Hum Mol Genet. 2011. PMID: 21257637 - Stress granule homeostasis is modulated by TRIM21-mediated ubiquitination of G3BP1 and autophagy-dependent elimination of stress granules.
Yang C, Wang Z, Kang Y, Yi Q, Wang T, Bai Y, Liu Y. Yang C, et al. Autophagy. 2023 Jul;19(7):1934-1951. doi: 10.1080/15548627.2022.2164427. Epub 2023 Jan 24. Autophagy. 2023. PMID: 36692217 Free PMC article. - Stress granules as crucibles of ALS pathogenesis.
Li YR, King OD, Shorter J, Gitler AD. Li YR, et al. J Cell Biol. 2013 Apr 29;201(3):361-72. doi: 10.1083/jcb.201302044. J Cell Biol. 2013. PMID: 23629963 Free PMC article. Review. - Stress granules at the intersection of autophagy and ALS.
Monahan Z, Shewmaker F, Pandey UB. Monahan Z, et al. Brain Res. 2016 Oct 15;1649(Pt B):189-200. doi: 10.1016/j.brainres.2016.05.022. Epub 2016 May 13. Brain Res. 2016. PMID: 27181519 Free PMC article. Review.
Cited by
- Therapeutic modulation of eIF2α phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models.
Kim HJ, Raphael AR, LaDow ES, McGurk L, Weber RA, Trojanowski JQ, Lee VM, Finkbeiner S, Gitler AD, Bonini NM. Kim HJ, et al. Nat Genet. 2014 Feb;46(2):152-60. doi: 10.1038/ng.2853. Epub 2013 Dec 15. Nat Genet. 2014. PMID: 24336168 Free PMC article. - TDP-43 regulates the alternative splicing of hnRNP A1 to yield an aggregation-prone variant in amyotrophic lateral sclerosis.
Deshaies JE, Shkreta L, Moszczynski AJ, Sidibé H, Semmler S, Fouillen A, Bennett ER, Bekenstein U, Destroismaisons L, Toutant J, Delmotte Q, Volkening K, Stabile S, Aulas A, Khalfallah Y, Soreq H, Nanci A, Strong MJ, Chabot B, Vande Velde C. Deshaies JE, et al. Brain. 2018 May 1;141(5):1320-1333. doi: 10.1093/brain/awy062. Brain. 2018. PMID: 29562314 Free PMC article. - Stress granule assembly in vivo is deficient in the CNS of mutant TDP-43 ALS mice.
Dubinski A, Gagné M, Peyrard S, Gordon D, Talbot K, Vande Velde C. Dubinski A, et al. Hum Mol Genet. 2023 Jan 6;32(2):319-332. doi: 10.1093/hmg/ddac206. Hum Mol Genet. 2023. PMID: 35994036 Free PMC article. - Erratum to: Endogenous TDP-43, but not FUS, contributes to stress granule assembly via G3BP.
Aulas A, Stabile S, Vande Velde C. Aulas A, et al. Mol Neurodegener. 2015 Sep 3;10:45. doi: 10.1186/s13024-015-0041-8. Mol Neurodegener. 2015. PMID: 26337162 Free PMC article. No abstract available. - Dysregulated molecular pathways in amyotrophic lateral sclerosis-frontotemporal dementia spectrum disorder.
Gao FB, Almeida S, Lopez-Gonzalez R. Gao FB, et al. EMBO J. 2017 Oct 16;36(20):2931-2950. doi: 10.15252/embj.201797568. Epub 2017 Sep 15. EMBO J. 2017. PMID: 28916614 Free PMC article. Review.
References
- Dion PA, Daoud H, Rouleau GA. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet. 2009;10:769–782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous