Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner - PubMed (original) (raw)
. 2012 Dec 15;189(12):5508-12.
doi: 10.4049/jimmunol.1202121. Epub 2012 Nov 9.
Ping-I Chiang, Christian Schmidt-Lauber, Sandhya Ganesan, William J Kaiser, Vijay A K Rathinam, Edward S Mocarski, Deepa Subramanian, Douglas R Green, Neal Silverman, Katherine A Fitzgerald, Ann Marshak-Rothstein, Eicke Latz
Affiliations
- PMID: 23144495
- PMCID: PMC3518757
- DOI: 10.4049/jimmunol.1202121
Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner
Lukas Bossaller et al. J Immunol. 2012.
Abstract
Fas, a TNF family receptor, is activated by the membrane protein Fas ligand expressed on various immune cells. Fas signaling triggers apoptosis and induces inflammatory cytokine production. Among the Fas-induced cytokines, the IL-1β family cytokines require proteolysis to gain biological activity. Inflammasomes, which respond to pathogens and danger signals, cleave IL-1β cytokines via caspase-1. However, the mechanisms by which Fas regulates IL-1β activation remain unresolved. In this article, we demonstrate that macrophages exposed to TLR ligands upregulate Fas, which renders them responsive to receptor engagement by Fas ligand. Fas signaling activates caspase-8 in macrophages and dendritic cells, leading to the maturation of IL-1β and IL-18 independently of inflammasomes or RIP3. Hence, Fas controls a novel noncanonical IL-1β activation pathway in myeloid cells, which could play an essential role in inflammatory processes, tumor surveillance, and control of infectious diseases.
Figures
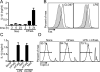
FIGURE 1. IL-1β activation by FAS is dependent on FAS receptor upregulation secondary to TLR priming in BMDMs
A, IL-1β ELISA of SN from BMDMs primed with LPS and stimulated for indicated times with Neo control or mFASL vesicles B, Histogram depicting the MFI of FAS on live-gated BMDM after 24h stimulation with 300 ng/ml CLO97 (line) or 20 ng/ml LPS (line) and untreated controls (tinted). C, IL-1β ELISA of SN from Neo or mFASL stimulated BMDMs, which were left untreated or primed for 24h with LPS or CLO97. D, Histograms of TOPRO-3 stained BMDMs in medium only (left), after addition of mFASL without priming (middle) or after 24h priming with LPS and stimulated with mFASL for 6h (right).
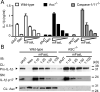
FIGURE 2. Inflammasome and caspase-1/11 independent IL-1β processing in BMDMs stimulated with mFASL
A, ELISA for IL-1β of SN from LPS-primed (24h) BMDMs from wt (white bars), ASC-/- (black bars) or Caspase-1/11-/- (grey bars) mice stimulated as indicated for an additional 6h with decreasing concentrations of mFAS-L, Neo or dAdT. B, Immunoblot of SN and cell lysates (CL) of wt and ASC-/- BMDMs of the same assay conditions as in A.
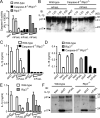
FIGURE 3. FAS-induced IL-1β and IL-18 processing is caspase-8 dependent and RIP3 independent
A, Caspase-8 activity in CL from wt, Caspase-8-/-/Rip3-/- or RIP3-/- BMDMs stimulated for 6h with decreasing concentrations of mFAS-L. B-F, Immunoblot (B,F) or ELISA for IL-1β (C,E) and IL-18 (D) of SN from wt, Caspase-8-/-/Rip3-/- or RIP3-/- BMDMs stimulated as indicated.
Similar articles
- Fas-mediated inflammatory response in Listeria monocytogenes infection.
Uchiyama R, Yonehara S, Tsutsui H. Uchiyama R, et al. J Immunol. 2013 Apr 15;190(8):4245-54. doi: 10.4049/jimmunol.1203059. Epub 2013 Mar 15. J Immunol. 2013. PMID: 23509366 - FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes.
Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. Gurung P, et al. J Immunol. 2014 Feb 15;192(4):1835-46. doi: 10.4049/jimmunol.1302839. Epub 2014 Jan 22. J Immunol. 2014. PMID: 24453255 Free PMC article. - TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo.
He Y, Franchi L, Núñez G. He Y, et al. J Immunol. 2013 Jan 1;190(1):334-9. doi: 10.4049/jimmunol.1202737. Epub 2012 Dec 7. J Immunol. 2013. PMID: 23225887 Free PMC article. - Caspase-11: the driving factor for noncanonical inflammasomes.
Viganò E, Mortellaro A. Viganò E, et al. Eur J Immunol. 2013 Sep;43(9):2240-5. doi: 10.1002/eji.201343800. Eur J Immunol. 2013. PMID: 24037676 Review. - Novel roles for caspase-8 in IL-1β and inflammasome regulation.
Gurung P, Kanneganti TD. Gurung P, et al. Am J Pathol. 2015 Jan;185(1):17-25. doi: 10.1016/j.ajpath.2014.08.025. Epub 2014 Nov 1. Am J Pathol. 2015. PMID: 25451151 Free PMC article. Review.
Cited by
- Inflammasome Complexes: Emerging Mechanisms and Effector Functions.
Rathinam VA, Fitzgerald KA. Rathinam VA, et al. Cell. 2016 May 5;165(4):792-800. doi: 10.1016/j.cell.2016.03.046. Cell. 2016. PMID: 27153493 Free PMC article. Review. - In vitro Evaluation of Programmed Cell Death in the Immune System of Pacific Oyster Crassostrea gigas by the Effect of Marine Toxins.
Estrada N, Núñez-Vázquez EJ, Palacios A, Ascencio F, Guzmán-Villanueva L, Contreras RG. Estrada N, et al. Front Immunol. 2021 Apr 1;12:634497. doi: 10.3389/fimmu.2021.634497. eCollection 2021. Front Immunol. 2021. PMID: 33868255 Free PMC article. - Porcine γδ T cells express cytotoxic cell-associated markers and display killing activity but are not selectively cytotoxic against PRRSV- or swIAV-infected macrophages.
Bettin L, Darbellay J, van Kessel J, Dhar N, Gerdts V. Bettin L, et al. Front Immunol. 2024 Jul 31;15:1434011. doi: 10.3389/fimmu.2024.1434011. eCollection 2024. Front Immunol. 2024. PMID: 39144143 Free PMC article. - Mechanisms of NOD-like receptor-associated inflammasome activation.
Wen H, Miao EA, Ting JP. Wen H, et al. Immunity. 2013 Sep 19;39(3):432-41. doi: 10.1016/j.immuni.2013.08.037. Immunity. 2013. PMID: 24054327 Free PMC article. Review. - IL-18 biology in severe asthma.
Thawanaphong S, Nair A, Volfson E, Nair P, Mukherjee M. Thawanaphong S, et al. Front Med (Lausanne). 2024 Nov 1;11:1486780. doi: 10.3389/fmed.2024.1486780. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39554494 Free PMC article. Review.
References
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. - PubMed
- Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. - PubMed
- Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rosen-Wolff A, Peters AM, Sneller MC, Hallahan CW, Wang J, Fischer RE, Jackson CM, Lin AY, Baumler C, Siegert E, Marx A, Vaishnaw AK, Grodzicky T, Fleisher TA, Lenardo MJ. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. - PubMed
- Fukuyama H, Adachi M, Suematsu S, Miwa K, Suda T, Yoshida N, Nagata S. Requirement of Fas expression in B cells for tolerance induction. Eur J Immunol. 2002;32:223–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI047891/AI/NIAID NIH HHS/United States
- R01 CA090691/CA/NCI NIH HHS/United States
- HL093262/HL/NHLBI NIH HHS/United States
- CA90691/CA/NCI NIH HHS/United States
- AR050256/AR/NIAMS NIH HHS/United States
- R01 AI060025/AI/NIAID NIH HHS/United States
- P01 AR050256/AR/NIAMS NIH HHS/United States
- AI057159/AI/NIAID NIH HHS/United States
- R01 HL093262/HL/NHLBI NIH HHS/United States
- R01 AI083713/AI/NIAID NIH HHS/United States
- AI083713/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous