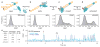Activated GTPase movement on an RNA scaffold drives co-translational protein targeting - PubMed (original) (raw)
Activated GTPase movement on an RNA scaffold drives co-translational protein targeting
Kuang Shen et al. Nature. 2012.
Abstract
Approximately one-third of the proteome is initially destined for the eukaryotic endoplasmic reticulum or the bacterial plasma membrane. The proper localization of these proteins is mediated by a universally conserved protein-targeting machinery, the signal recognition particle (SRP), which recognizes ribosomes carrying signal sequences and, through interactions with the SRP receptor, delivers them to the protein-translocation machinery on the target membrane. The SRP is an ancient ribonucleoprotein particle containing an essential, elongated SRP RNA for which precise functions have remained elusive. Here we used single-molecule fluorescence microscopy to show that the Escherichia coli SRP-SRP receptor GTPase complex, after initial assembly at the tetraloop end of SRP RNA, travels over 100 Å to the distal end of this RNA, where rapid GTP hydrolysis occurs. This movement is negatively regulated by the translating ribosome and, at a later stage, positively regulated by the SecYEG translocon, providing an attractive mechanism for ensuring the productive exchange of the targeting and translocation machineries at the ribosome exit site with high spatial and temporal accuracy. Our results show that large RNAs can act as molecular scaffolds that enable the easy exchange of distinct factors and precise timing of molecular events in a complex cellular process; this concept may be extended to similar phenomena in other ribonucleoprotein complexes.
Figures
Figure 1. smFRET-TIRF microscopy reveals dynamic movements of the SRP-FtsY complex on the SRP RNA
a, smFRET setup for the SRP-FtsY complex. FtsY C345 is labeled with Cy3. The 5′-end of the DNA splint (2 nt from the 3′-end of SRP RNA) is labeled with Quasar670. b, Fluorescent signals (upper) and FRET trajectory (lower) of the SRP-FtsY complex in GMPPNP. Hidden Markov Modeling (HMM) of the FRET trajectory is in navy. The arrow denotes the bleaching of Cy3, after which Quasar670 was excited using a 635nm laser to confirm the presence of the complex. c, Magnification of the grey box in b to depict the four FRET states resolved by HMM. d, smFRET histogram depicting the distribution of molecules in different states. In M state, the M1 and M2 states are binned together. e, Transition density plot (TDP) for the GTPase movements. f, Analysis of the transition kinetics between L and H states. Exponential fits of the data gave the transition rate constants in g.
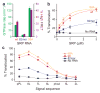
Figure 2. The distal site of SRP RNA is crucial for GTPase activation and protein targeting
a, Correlation between GTPase rate constants in the SRP-FtsY complex (green bars) and the frequency of reaching the high FRET state (purple bars) for wildtype (red), 82mer (blue), and 99A SRP RNA (orange). Data represent mean±s.d. (n=5). b–c, Co-translational targeting and translocation of pPL (b) and its signal sequence variants (c) mediated by the wildtype and mutant SRPs. Color codings are the same as in a. Reactions in the absence of SRP RNA are in black. Data represent mean±s.d. (n=3).
Figure 3. Conformational rearrangements within the SRP-FtsY GTPase complex drive its movement to the RNA distal site
a–d, smFRET histograms of free SRP in the open state (a) and of the SRP-FtsY complex in the early (b), closed (c), and activated (d) states. Conditions for isolating each conformational state are described in the text and Methods. e, Summary of the FRET distributions. f, A representative smFRET trajectory of the complex incubated in GTP. The arrow denotes a burst of high FRET that results from GTPase docking at the distal site terminated by rapid GTP hydrolysis driving complex disassembly. Δτ denotes the duration of the high FRET burst.
Figure 4. RNC and SecYEG regulate GTPase movements on the SRP RNA
a, Effect of RNCFtsQ (grey bars) and SecYEG (purple bars) on the GTPase activity of the SRP-FtsY complex assembled with the wildtype, 82mer, and 99A SRP RNA. Data represent mean±s.d. (n=3). b–c, smFRET histograms of the SRP-FtsY complex bound to RNCFtsQ (b) or RNCLuciferase (c). d, Fluorescent signals (upper) and FRET trajectory (lower) of the RNCFtsQ-SRP-FtsY complex in the presence of SecYEG. Color coding is the same as in Fig. 1b. e–f, smFRET histograms of the RNCFtsQ-SRP-FtsY complex in the presence of SecYEG with the wildtype (e) or 82mer (f) SRP RNA. g, TDP of the GTPase movements in the presence of RNCFtsQ and SecYEG. h, Summary of the percentage of molecules that exhibit the specified transitions. In the presence of RNCFtsQ and SecYEG, transitions to intermediate FRET states are significantly reduced. i, Scatter plot of the transition dwell times of individual molecules in the absence (grey circles) and presence of RNCFtsQ and SecYEG (red crosses). j, Model for the role of the SRP RNA-mediated GTPase relocalization in co-translational protein targeting.
Comment in
- Cell biology: All clear for ribosome landing.
Bernstein HD. Bernstein HD. Nature. 2012 Dec 13;492(7428):189-91. doi: 10.1038/492189a. Nature. 2012. PMID: 23235870 Free PMC article. No abstract available.
Similar articles
- Molecular mechanism of GTPase activation at the signal recognition particle (SRP) RNA distal end.
Shen K, Wang Y, Hwang Fu YH, Zhang Q, Feigon J, Shan SO. Shen K, et al. J Biol Chem. 2013 Dec 20;288(51):36385-97. doi: 10.1074/jbc.M113.513614. Epub 2013 Oct 22. J Biol Chem. 2013. PMID: 24151069 Free PMC article. - The crystal structure of the signal recognition particle in complex with its receptor.
Ataide SF, Schmitz N, Shen K, Ke A, Shan SO, Doudna JA, Ban N. Ataide SF, et al. Science. 2011 Feb 18;331(6019):881-6. doi: 10.1126/science.1196473. Science. 2011. PMID: 21330537 Free PMC article. - SRP RNA provides the physiologically essential GTPase activation function in cotranslational protein targeting.
Siu FY, Spanggord RJ, Doudna JA. Siu FY, et al. RNA. 2007 Feb;13(2):240-50. doi: 10.1261/rna.135407. Epub 2006 Dec 12. RNA. 2007. PMID: 17164479 Free PMC article. - Co-translational protein targeting to the bacterial membrane.
Saraogi I, Shan SO. Saraogi I, et al. Biochim Biophys Acta. 2014 Aug;1843(8):1433-41. doi: 10.1016/j.bbamcr.2013.10.013. Epub 2013 Oct 24. Biochim Biophys Acta. 2014. PMID: 24513458 Free PMC article. Review. - Co-translational protein targeting by the signal recognition particle.
Shan SO, Walter P. Shan SO, et al. FEBS Lett. 2005 Feb 7;579(4):921-6. doi: 10.1016/j.febslet.2004.11.049. FEBS Lett. 2005. PMID: 15680975 Review.
Cited by
- ATPase and GTPase Tangos Drive Intracellular Protein Transport.
Shan SO. Shan SO. Trends Biochem Sci. 2016 Dec;41(12):1050-1060. doi: 10.1016/j.tibs.2016.08.012. Epub 2016 Sep 19. Trends Biochem Sci. 2016. PMID: 27658684 Free PMC article. Review. - Spatial and temporal organization of RecA in the Escherichia coli DNA-damage response.
Ghodke H, Paudel BP, Lewis JS, Jergic S, Gopal K, Romero ZJ, Wood EA, Woodgate R, Cox MM, van Oijen AM. Ghodke H, et al. Elife. 2019 Feb 5;8:e42761. doi: 10.7554/eLife.42761. Elife. 2019. PMID: 30717823 Free PMC article. - Ribosome binding induces repositioning of the signal recognition particle receptor on the translocon.
Kuhn P, Draycheva A, Vogt A, Petriman NA, Sturm L, Drepper F, Warscheid B, Wintermeyer W, Koch HG. Kuhn P, et al. J Cell Biol. 2015 Oct 12;211(1):91-104. doi: 10.1083/jcb.201502103. J Cell Biol. 2015. PMID: 26459600 Free PMC article. - A Dynamic Search Process Underlies MicroRNA Targeting.
Chandradoss SD, Schirle NT, Szczepaniak M, MacRae IJ, Joo C. Chandradoss SD, et al. Cell. 2015 Jul 2;162(1):96-107. doi: 10.1016/j.cell.2015.06.032. Cell. 2015. PMID: 26140593 Free PMC article. - Take Me Home, Protein Roads: Structural Insights into Signal Peptide Interactions during ER Translocation.
Liaci AM, Förster F. Liaci AM, et al. Int J Mol Sci. 2021 Nov 1;22(21):11871. doi: 10.3390/ijms222111871. Int J Mol Sci. 2021. PMID: 34769302 Free PMC article. Review.
References
- Keenan RJ, Freymann DM, Stroud RM, Walter P. The signal recognition particle. Annu Rev Biochem. 2001;70:755–775. - PubMed
- Pool MR, Stumm J, Fulga TA, Sinning I, Dobberstein B. Distinct modes of signal recognition particle interaction with the ribosome. Science. 2002;297:1345–1348. - PubMed
- Halic M, et al. Following the signal sequence from ribosomal tunnel exit to signal recognition particle. Nature. 2006;444:507–511. - PubMed
- Schaffitzel C, et al. Structure of the E-coli signal recognition particle bound to a translating ribosome. Nature. 2006;444:503–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM045162/GM/NIGMS NIH HHS/United States
- GM065367/GM/NIGMS NIH HHS/United States
- GM45162/GM/NIGMS NIH HHS/United States
- R01 GM065367/GM/NIGMS NIH HHS/United States
- GM078024/GM/NIGMS NIH HHS/United States
- R37 GM045162/GM/NIGMS NIH HHS/United States
- R01 GM078024/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases