Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor - PubMed (original) (raw)
Clinical Trial
. 2013 Feb 1;31(4):482-9.
doi: 10.1200/JCO.2012.43.5966. Epub 2012 Dec 17.
Richard Kefford, Anna C Pavlick, Jeffrey R Infante, Antoni Ribas, Jeffrey A Sosman, Leslie A Fecher, Michael Millward, Grant A McArthur, Patrick Hwu, Rene Gonzalez, Patrick A Ott, Georgina V Long, Olivia S Gardner, Daniele Ouellet, Yanmei Xu, Douglas J DeMarini, Ngocdiep T Le, Kiran Patel, Karl D Lewis
Affiliations
- PMID: 23248257
- PMCID: PMC4878037
- DOI: 10.1200/JCO.2012.43.5966
Clinical Trial
Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor
Kevin B Kim et al. J Clin Oncol. 2013.
Abstract
Purpose: BRAF mutations promote melanoma cell proliferation and survival primarily through activation of MEK. The purpose of this study was to determine the response rate (RR) for the selective, allosteric MEK1/MEK2 inhibitor trametinib (GSK1120212), in patients with metastatic BRAF-mutant melanoma.
Patients and methods: This was an open-label, two-stage, phase II study with two cohorts. Patients with metastatic BRAF-mutant melanoma previously treated with a BRAF inhibitor (cohort A) or treated with chemotherapy and/or immunotherapy (BRAF-inhibitor naive; cohort B) were enrolled. Patients received 2 mg of trametinib orally once daily.
Results: In cohort A (n = 40), there were no confirmed objective responses and 11 patients (28%) with stable disease (SD); the median progression-free survival (PFS) was 1.8 months. In cohort B (n = 57), there was one (2%) complete response, 13 (23%) partial responses (PRs), and 29 patients (51%) with SD (confirmed RR, 25%); the median PFS was 4.0 months. One patient each with BRAF K601E and BRAF V600R had prolonged PR. The most frequent treatment-related adverse events for all patients were skin-related toxicity, nausea, peripheral edema, diarrhea, pruritis, and fatigue. No cutaneous squamous cell carcinoma was observed.
Conclusion: Trametinib was well tolerated. Significant clinical activity was observed in BRAF-inhibitor-naive patients previously treated with chemotherapy and/or immunotherapy. Minimal clinical activity was observed as sequential therapy in patients previously treated with a BRAF inhibitor. Together, these data suggest that BRAF-inhibitor resistance mechanisms likely confer resistance to MEK-inhibitor monotherapy. These data support further evaluation of trametinib in BRAF-inhibitor-naive BRAF-mutant melanoma, including rarer forms of BRAF-mutant melanoma.
Trial registration: ClinicalTrials.gov NCT01037127.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
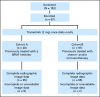
Fig 1.
Trial design.
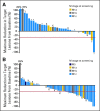
Fig 2.
Best percentage change from baseline in target lesions for (A) cohort A (radiographic scan data was incomplete or unavailable for 5 patients) and (B) cohort B (radiographic scan data was incomplete or unavailable for two patients). Staging was according to the American Joint Committee on Cancer (AJCC) system. (*) Patients with prior brain metastases; (†) patients who discontinued prior BRAF inhibitor because of toxicity; (K) patients with the V600K mutation.
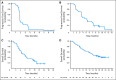
Fig 3.
Kaplan-Meier estimated survival for all patients by cohort: (A) progression-free survival (PFS) for cohort A, (B) PFS for cohort B, (C) overall survival (OS) for cohort A, and (D) OS for cohort B. The bars represent the date of the last adequate tumor assessment before the data cutoff among patients with censored data without disease progression or death.
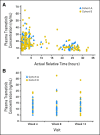
Fig A1.
Pharmacokinetic analysis. (A) Individual plasma trametinib concentration versus actual time postdose observed on day 15. (B) Individual plasma predose trametinib concentration observed at weeks 4, 8, and 12.
Similar articles
- Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial.
Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, DeMarini DJ, Sun P, Moy C, Szabo SA, Roadcap LT, Peddareddigari VG, Lebowitz PF, Le NT, Burris HA 3rd, Messersmith WA, O'Dwyer PJ, Kim KB, Flaherty K, Bendell JC, Gonzalez R, Kurzrock R, Fecher LA. Falchook GS, et al. Lancet Oncol. 2012 Aug;13(8):782-9. doi: 10.1016/S1470-2045(12)70269-3. Epub 2012 Jul 16. Lancet Oncol. 2012. PMID: 22805292 Free PMC article. Clinical Trial. - Improved survival with MEK inhibition in BRAF-mutated melanoma.
Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, Dummer R, Trefzer U, Larkin JM, Utikal J, Dreno B, Nyakas M, Middleton MR, Becker JC, Casey M, Sherman LJ, Wu FS, Ouellet D, Martin AM, Patel K, Schadendorf D; METRIC Study Group. Flaherty KT, et al. N Engl J Med. 2012 Jul 12;367(2):107-14. doi: 10.1056/NEJMoa1203421. Epub 2012 Jun 4. N Engl J Med. 2012. PMID: 22663011 Clinical Trial. - A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)†.
Blumenschein GR Jr, Smit EF, Planchard D, Kim DW, Cadranel J, De Pas T, Dunphy F, Udud K, Ahn MJ, Hanna NH, Kim JH, Mazieres J, Kim SW, Baas P, Rappold E, Redhu S, Puski A, Wu FS, Jänne PA. Blumenschein GR Jr, et al. Ann Oncol. 2015 May;26(5):894-901. doi: 10.1093/annonc/mdv072. Epub 2015 Feb 26. Ann Oncol. 2015. PMID: 25722381 Free PMC article. Clinical Trial. - Role of the MEK inhibitor trametinib in the treatment of metastatic melanoma.
King JW, Nathan PD. King JW, et al. Future Oncol. 2014;10(9):1559-70. doi: 10.2217/fon.14.89. Future Oncol. 2014. PMID: 25145427 Review. - Trametinib (MEKINIST°) Metastatic or inoperable BRAF V600-positive melanoma: a few extra months of life.
[No authors listed] [No authors listed] Prescrire Int. 2016 Dec;25(177):285-288. Prescrire Int. 2016. PMID: 30758923 Review.
Cited by
- Mutated BRAF Emerges as a Major Effector of Recurrence in a Murine Melanoma Model After Treatment With Immunomodulatory Agents.
Zaidi S, Blanchard M, Shim K, Ilett E, Rajani K, Parrish C, Boisgerault N, Kottke T, Thompson J, Celis E, Pulido J, Selby P, Pandha H, Melcher A, Harrington K, Vile R. Zaidi S, et al. Mol Ther. 2015 May;23(5):845-856. doi: 10.1038/mt.2014.253. Epub 2014 Dec 29. Mol Ther. 2015. PMID: 25544599 Free PMC article. - BRAF-mutant melanoma: treatment approaches, resistance mechanisms, and diagnostic strategies.
Spagnolo F, Ghiorzo P, Orgiano L, Pastorino L, Picasso V, Tornari E, Ottaviano V, Queirolo P. Spagnolo F, et al. Onco Targets Ther. 2015 Jan 16;8:157-68. doi: 10.2147/OTT.S39096. eCollection 2015. Onco Targets Ther. 2015. PMID: 25653539 Free PMC article. Review. - Dabrafenib plus Trametinib: a Review in Advanced Melanoma with a BRAF (V600) Mutation.
Dhillon S. Dhillon S. Target Oncol. 2016 Jun;11(3):417-28. doi: 10.1007/s11523-016-0443-8. Target Oncol. 2016. PMID: 27246822 Review. - A recommended practical approach to the management of target therapy and angiogenesis inhibitors cardiotoxicity: an opinion paper of the working group on drug cardiotoxicity and cardioprotection, Italian Society of Cardiology.
Maurea N, Spallarossa P, Cadeddu C, Madonna R, Mele D, Monte I, Novo G, Pagliaro P, Pepe A, Tocchetti CG, Zito C, Mercuro G. Maurea N, et al. J Cardiovasc Med (Hagerstown). 2016 May;17 Suppl 1(Suppl 1):e93-e104. doi: 10.2459/JCM.0000000000000383. J Cardiovasc Med (Hagerstown). 2016. PMID: 27183530 Free PMC article. Review. - Reasons for non-feasibility of therapeutic drug monitoring of oral targeted therapies in oncology - an analysis of the closed cohorts of a multicentre prospective study.
van der Kleij MBA, Guchelaar NAD, Meertens M, Westerdijk K, Giraud EL, Bleckman RF, Groenland SL, van Eerden RAG, Imholz ALT, Vulink AJE, Otten HM, Fiebrich-Westra HB, Lubberman FJE, Desar IME, Moes DAR, Touw DJ, Koolen SLW, Gelderblom H, Reyners AKL, van Erp NP, Mathijssen RHJ, Huitema ADR; Dutch Pharmacology Oncology Group (DPOG); Steeghs N. van der Kleij MBA, et al. Br J Cancer. 2024 Sep;131(5):843-851. doi: 10.1038/s41416-024-02789-2. Epub 2024 Jul 6. Br J Cancer. 2024. PMID: 38971952 Free PMC article.
References
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. - PubMed
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–1246. - PubMed
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991–997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous