Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock-out mice following stroke - PubMed (original) (raw)
Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock-out mice following stroke
Matthias W Sieber et al. PLoS One. 2013.
Abstract
Background: Triggering receptor expressed on myeloid cells-2 (TREM2) is a microglial surface receptor involved in phagocytosis. Clearance of apoptotic debris after stroke represents an important mechanism to re-attain tissue homeostasis and thereby ensure functional recovery. The role of TREM2 following stroke is currently unclear.
Methods and results: As an experimental stroke model, the middle cerebral artery of mice was occluded for 30 minutes with a range of reperfusion times (duration of reperfusion: 6 h/12 h/24 h/2 d/7 d/28 d). Quantitative PCR (qPCR) revealed a greatly increased transcription of TREM2 after stroke. We subsequently analyzed the expression of pro-inflammatory cytokines, chemokines and their receptors in TREM2-knockout (TREM2-KO) mice via qPCR. Microglial activation (CD68, Iba1) and CD3-positive T-cell invasion were analyzed via qPCR and immunohistochemistry. Functional consequences of TREM2 knockout were assessed by infarct volumetry. The acute inflammatory response (12 h reperfusion) was very similar between TREM2-KO mice and their littermate controls. However, in the sub-acute phase (7 d reperfusion) following stroke, TREM2-KO mice showed a decreased transcription of pro-inflammatory cytokines TNFα, IL-1α and IL-1β, associated with a reduced microglial activity (CD68, Iba1). Furthermore, TREM2-KO mice showed a reduced transcription of chemokines CCL2 (MCP1), CCL3 (MIP1α) and the chemokine receptor CX3CR1, followed by a diminished invasion of CD3-positive T-cells. No effect on the lesion size was observed.
Conclusions: Although we initially expected an exaggerated pro-inflammatory response following ablation of TREM2, our data support a contradictory scenario that the sub-acute inflammatory reaction after stroke is attenuated in TREM2-KO mice. We therefore conclude that TREM2 appears to sustain a distinct inflammatory response after stroke.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
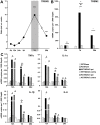
Figure 1. Expression of TREM2 and pro-inflammatory cytokine transcripts in C57BL/6 and TREM2-KO mice after stroke.
A. Gene transcripts for TREM2 were determined at 6 h, 12 h, 24 h, 2 d, 7 d and 28 d after stroke in the ipsilateral ischemic hemisphere of C57BL/6 mice. TREM2 was up-regulated at 7 d and 28 d after stroke. Data are displayed as mean ± s.e.m. (ratio ipsi vs. contra), n = 4 each, ***p≤0.001. B. TREM2 gene transcripts were determined at 12 h, 7 d and 28 d after stroke (MCAO) as well as after sham procedure (Sham) in TREM2-KO mice (KO) and littermate controls (WT). Increased TREM2 gene transcription was detected in the ischemic hemisphere of littermate controls (WT MCAO ipsi) at 7 d and 28 d. No increased gene transcripts for TREM2 were observed after stroke in littermate controls at the contralateral side (WT MCAO contra) and following sham procedure (WT Sham). TREM2-KO mice (KO) did not show any TREM2 gene transcription. C. Gene transcripts of cytokines were determined after stroke (MCAO) as well as after sham procedure (Sham) in TREM2-KO mice (KO) and littermate controls (WT). A strong up-regulation of pro-inflammatory cytokines (TNFα, IL-1α, IL-1β and IL-6) was observed 12 h after stroke. A reduced gene transcription of TNFα, IL-1α and IL-1β was observed in TREM2-KO (KO MCAO ipsi) compared to littermate control mice (WT MCAO ipsi) at 7 d after stroke. No differences in cytokine gene transcription were revealed between TREM2-KO mice (KO MCAO ipsi) and littermate controls (WT MCAO ipsi) at 12 h and at 28 d. No increased cytokine expression was observed after stroke in littermate controls at the contralateral side (contra) and in sham operated mice (Sham). Bars represent mean ± s.e.m. (mRNA copies per 1,000 Gapdh), sham: n = 3 each and MCAO: n = 5/6 each, WT ipsi vs. WT contra #p≤0.05, ##p≤0.01, WT ipsi vs. KO ipsi *p≤0.05, **p≤0.01, ***p≤0.001.
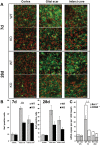
Figure 2. Decreased microglial activation in TREM2-KO mice after stroke.
A. Post-ischemic Iba1 positive cells (green) displayed the typical activated amoeboid phenotype in the glial scar and infarct core at 7 d and 28 d in WT mice, whereas they remained in a ramified phenotype in TREM2-KO mice. Nuclei of cells counterstained with DAPI (for a better distinction the color was changed to red). Scale bar 20 µm. B. Fewer Iba1 positive activated microglial cells were revealed in the glial scar of TREM2-KO mice (KO) at 7 d following stroke compared to littermate controls (WT). Bars represent mean ± s.e.m., n = 5/6 each, WT ipsi vs. KO ipsi ***p≤0.001. C. Gene transcripts for Iba1 and CD68 were determined at 12 h, 7 d and 28 d after stroke in TREM2-KO mice (KO) and littermate controls (WT). Attenuated gene transcription of Iba1 and CD68 was observed 7 d after stroke in TREM2-KO (KO) mice compared to control (WT) mice. Bars represent mean ± s.e.m. (mRNA copies per 1,000 Gapdh), n = 5/6 each, WT ipsi vs. KO ipsi *p≤0.05 (Iba1), +p≤0.05 (CD68).
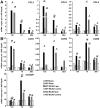
Figure 3. Decreased chemokine and chemokine receptor gene transcription in TREM2-KO mice after stroke.
A. The mRNAs for the chemokines CCL3, CCL2 and CCL5 were increased following stroke in littermate controls (WT) as well as in TREM2-KO mice, with a peak at 12 h for CCL3 and CCL2 and at 7 d for CCL5. Reduced gene transcription of CCL3 and CCL2 was observed at 7 d after stroke in TREM2-KO mice compared to littermate controls. No increased chemokine expression was observed after stroke in littermate controls at the contralateral side and in sham-operated mice. B. Gene transcripts of chemokine receptors were increased after stroke, with a peak at 12 h for CCR1 and at 7 d for CCR2, CCR5 and CX3CR1. Chemokine receptor CX3CR1 expression was attenuated following stroke in TREM2-KO mice. Post-ischemic expression of chemokine receptors was unchanged in littermate controls at the contralateral side and in sham-operated mice. Bars represent mean ± s.e.m. (mRNA copies per 1,000 Gapdh), sham: n = 3 each and MCAO: n = 5/6 each, WT ipsi vs. WT contra #p≤0.05, ##p≤0.01, ###p≤0.001, WT ipsi vs. KO ipsi *p≤0.05, **p≤0.01.
Figure 4. Fewer CD3-positive T-cells in TREM2-KO mice after stroke.
A. CD3-positive T-cells invaded the infarct core at 7 d and 28 d following stroke. Increased numbers of CD3-positive T-cells were visible in the infarct core 28 d after stroke in littermate controls, but not in TREM2-KO mice. Scale bar 50 µm. B. Fewer CD3-positive T-cells were detected 28 d after stroke in TREM2-KO mice compared to their littermate controls. Bars represent mean ± s.e.m, n = 3 each, WT ipsi vs. KO ipsi *p≤0.05.
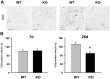
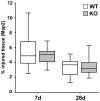
Figure 5. No change in brain tissue injury in TREM2-KO mice after stroke.
Infarct volumes of TREM2-KO and littermate control mice were analyzed at 7 d and 28 d based on Map2 immunohistochemistry. TREM2-KO mice and littermate control mice showed the same relative loss in Map2 immunostaining as a sign of tissue injury. Box and whisker plots represent mean ± s.e.m. (mm3 injured tissue vs. ipsilateral hemisphere in %), n = 9–14 each.
Similar articles
- Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke.
Kawabori M, Kacimi R, Kauppinen T, Calosing C, Kim JY, Hsieh CL, Nakamura MC, Yenari MA. Kawabori M, et al. J Neurosci. 2015 Feb 25;35(8):3384-96. doi: 10.1523/JNEUROSCI.2620-14.2015. J Neurosci. 2015. PMID: 25716838 Free PMC article. - Triggering receptor expressed on myeloid cells-2 expression in the brain is required for maximal phagocytic activity and improved neurological outcomes following experimental stroke.
Kurisu K, Zheng Z, Kim JY, Shi J, Kanoke A, Liu J, Hsieh CL, Yenari MA. Kurisu K, et al. J Cereb Blood Flow Metab. 2019 Oct;39(10):1906-1918. doi: 10.1177/0271678X18817282. Epub 2018 Dec 7. J Cereb Blood Flow Metab. 2019. PMID: 30523715 Free PMC article. - TREM2 protects against cerebral ischemia/reperfusion injury.
Wu R, Li X, Xu P, Huang L, Cheng J, Huang X, Jiang J, Wu LJ, Tang Y. Wu R, et al. Mol Brain. 2017 Jun 7;10(1):20. doi: 10.1186/s13041-017-0296-9. Mol Brain. 2017. PMID: 28592261 Free PMC article. - TREM2, microglial and ischemic stroke.
Wang H, Li X, Wang Q, Ma J, Gao X, Wang M. Wang H, et al. J Neuroimmunol. 2023 Aug 15;381:578108. doi: 10.1016/j.jneuroim.2023.578108. Epub 2023 May 16. J Neuroimmunol. 2023. PMID: 37302170 Review. - The Emerging Role of Triggering Receptor Expressed on Myeloid Cells 2 as a Target for Immunomodulation in Ischemic Stroke.
Gervois P, Lambrichts I. Gervois P, et al. Front Immunol. 2019 Jul 17;10:1668. doi: 10.3389/fimmu.2019.01668. eCollection 2019. Front Immunol. 2019. PMID: 31379859 Free PMC article. Review.
Cited by
- TREM2 regulates microglial cell activation in response to demyelination in vivo.
Cantoni C, Bollman B, Licastro D, Xie M, Mikesell R, Schmidt R, Yuede CM, Galimberti D, Olivecrona G, Klein RS, Cross AH, Otero K, Piccio L. Cantoni C, et al. Acta Neuropathol. 2015 Mar;129(3):429-47. doi: 10.1007/s00401-015-1388-1. Epub 2015 Jan 29. Acta Neuropathol. 2015. PMID: 25631124 Free PMC article. - Phagocytic microglia and macrophages in brain injury and repair.
Yu F, Wang Y, Stetler AR, Leak RK, Hu X, Chen J. Yu F, et al. CNS Neurosci Ther. 2022 Sep;28(9):1279-1293. doi: 10.1111/cns.13899. Epub 2022 Jun 25. CNS Neurosci Ther. 2022. PMID: 35751629 Free PMC article. Review. - Triggering Receptor Expressed on Myeloid Cells-2 Correlates to Hypothermic Neuroprotection in Ischemic Stroke.
Kawabori M, Hokari M, Zheng Z, Kim JY, Calosing C, Hsieh CL, Nakamura MC, Yenari MA. Kawabori M, et al. Ther Hypothermia Temp Manag. 2013 Dec 1;3(4):189-198. doi: 10.1089/ther.2013.0020. Ther Hypothermia Temp Manag. 2013. PMID: 24380032 Free PMC article. - The triggering receptor expressed on myeloid cells 2 (TREM2) is associated with enhanced inflammation, neuropathological lesions and increased risk for Alzheimer's dementia.
Roussos P, Katsel P, Fam P, Tan W, Purohit DP, Haroutunian V. Roussos P, et al. Alzheimers Dement. 2015 Oct;11(10):1163-70. doi: 10.1016/j.jalz.2014.10.013. Epub 2014 Dec 9. Alzheimers Dement. 2015. PMID: 25499537 Free PMC article. - TREM2-Ligand Interactions in Health and Disease.
Kober DL, Brett TJ. Kober DL, et al. J Mol Biol. 2017 Jun 2;429(11):1607-1629. doi: 10.1016/j.jmb.2017.04.004. Epub 2017 Apr 19. J Mol Biol. 2017. PMID: 28432014 Free PMC article. Review.
References
- Kunz A, Dirnagl U, Mergenthaler P (2010) Acute pathophysiological processes after ischaemic and traumatic brain injury. Best Pract Res Clin Anaesthesiol 24: 495–509. - PubMed
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91: 461–553. - PubMed
- Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT (2009) Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J 276: 13–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This project was supported by the European Union (STREP, FP6, ‘Age-dependent inflammatory response after stroke’ – ARGES [LSHB-CT-2006-018936]) and is part of the research program of the Jena centre for systems biology of ageing – Jenage (BMBF 0315581). The Neural Regeneration Group at the University Bonn LIFE & BRAIN Center is supported by the Hertie-Foundation and the Deutsche Forschungsgemeinschaft (KFO177, SFB704, FOR1336). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials