Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation - PubMed (original) (raw)
Review
Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation
Matthias Wacker et al. Nutrients. 2013.
Abstract
Vitamin D, the sunshine vitamin, has received a lot of attention recently as a result of a meteoric rise in the number of publications showing that vitamin D plays a crucial role in a plethora of physiological functions and associating vitamin D deficiency with many acute and chronic illnesses including disorders of calcium metabolism, autoimmune diseases, some cancers, type 2 diabetes mellitus, cardiovascular disease and infectious diseases. Vitamin D deficiency is now recognized as a global pandemic. The major cause for vitamin D deficiency is the lack of appreciation that sun exposure has been and continues to be the major source of vitamin D for children and adults of all ages. Vitamin D plays a crucial role in the development and maintenance of a healthy skeleton throughout life. There remains some controversy regarding what blood level of 25-hydroxyvitamin D should be attained for both bone health and reducing risk for vitamin D deficiency associated acute and chronic diseases and how much vitamin D should be supplemented.
Figures
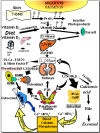
Figure 1
Schematic representation of the synthesis and metabolism of vitamin D for regulating calcium, phosphorus and bone metabolism [7]. During exposure to sunlight, 7-dehydrocholesterol in the skin is converted to previtamin D3. Previtamin D3 immediately converts by a heat dependent process to vitamin D3 [7,11,12]. Excessive exposure to sunlight degrades previtamin D3 and vitamin D3 into inactive photoproducts [13]. Vitamin D2 and vitamin D3 from dietary sources is incorporated into chylomicrons, transported by the lymphatic system into the venous circulation [14]. Vitamin D (D represents D2 or D3) made in the skin or ingested in the diet can be stored in and then released from fat cells. Vitamin D in the circulation is bound to the vitamin D binding protein which transports it to the liver where vitamin D is converted by the vitamin D-25-hydroxylase to 25-hydroxyvitamin D [25(OH)D]. This is the major circulating form of vitamin D that is used by clinicians to measure vitamin D status [7,15] (although most reference laboratories report the normal range to be 20–100 ng/mL, the preferred healthful range is 30–60 ng/mL) [7]. It is biologically inactive and must be converted in the kidneys by the 25-hydroxyvitamin D-1α-hydroxylase (1-OHase) to its biologically active form 1,25-dihydroxyvitamin D [1,25(OH)2D] [7,15,16,17]. Serum phosphorus, calcium, fibroblast growth factors (FGF-23) and other factors can either increase (+) or decrease (−) the renal production of 1,25(OH)2D [7]. 1,25(OH)2D feedback regulates its own synthesis and decreases the synthesis and secretion of parathyroid hormone (PTH) in the parathyroid glands [6,7]. 1,25(OH)2D increases the expression of the 25-hydroxyvitamin D-24-hydroxylase (24-OHase) to catabolize 1,25(OH)2D to the water soluble biologically inactive calcitroic acid which is excreted in the bile [7,18]. 1,25(OH)2D enhances intestinal calcium absorption in the small intestine by stimulating the expression of the epithelial calcium channel (ECaC) and the calbindin 9K (calcium binding protein; CaBP) [7,19,20]. 1,25(OH)2D is recognized by its receptor in osteoblasts causing an increase in the expression of receptor activator of NFκB ligand (RANKL). Its receptor RANK on the preosteoclast binds RANKL which induces the preosteoclast to become a mature osteoclast. The mature osteoclast removes calcium and phosphorus from the bone to maintain blood calcium and phosphorus levels [7,17]. Adequate calcium and phosphorus levels promote the mineralization of the skeleton [7]. Note: This figure is reproduced with permission from [21], Copyright © 2007 Michael F. Holick.
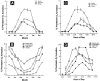
Figure 2
Influence of season, time of day, and latitude on the synthesis of previtamin D3 in Northern (A and C) and Southern hemispheres (B and D). The hour indicated in C and D is the end of the 1-h exposure time. Note: This figure is reproduced with permission from [13], Copyright © 2010 Humana Press.
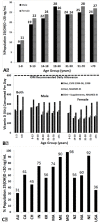
Figure 3
(A) Prevalence at risk of vitamin D deficiency defined as a 25-hydroxyvitamin D <12–20 ng/mL by age and sex: United States, 2001–2006. (B) Mean intake of vitamin D (IU) from food and food plus dietary supplements from Continuing Survey of Food Intakes by Individuals (CSFII) 1994–1996, 1998 and the Third National Health and Nutrition Examination Survey (NHANES III) 1988–1994. (C) Reported incidence of vitamin D deficiency defined as a 25-hydroxyvitamin D <20 ng/mL around the globe including Australia (AU), Canada (CA), China (CH), India (IN), Korea (KR), Malaysia (MA), Middle East (ME), Mongolia (MO), New Zealand (NZ), North Africa (NA), Northern Europe (NE), United States (USA) [60]. Note: This figure is reproduced with permission from [60], Copyright © 2012 The Endocrine Society.
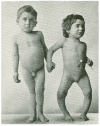
Figure 4
Sister (right) and brother (left) ages 4 years and 6.5 years, respectively, demonstrating classic knock-knees and bow legs, growth retardation, and other skeletal deformities [14]. Note: This figure is reproduced with permission from [14], Copyright © 2006 American Society for Clinical Investigation.
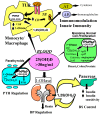
Figure 5
Metabolism of 25-hydroxyvitamin D [25(OH)D] to 1,25 dihydroxyvitamin D 1,25(OH)2D for non-skeletal functions. When a monocyte/macrophage is stimulated through its toll-like receptor 2/1 (TLR2/1) by an infective agent such as Mycobacterium tuberculosis (TB), or its lipopolysaccharide (LPS) the signal upregulates the expression of vitamin D receptor (VDR) and the 25-hydroxyvitamin D-1-hydroxylase (1-OHase). 25(OH)D levels >30 ng/mL provides adequate substrate for the 1-OHase to convert it to 1,25(OH)2D. 1,25(OH)2D returns to the nucleus where it increases the expression of cathelicidin which is a peptide capable of promoting innate immunity and inducing the destruction of infective agents such as TB. It is also likely that the 1,25(OH)2D produced in the monocytes/macrophage is released to act locally on activated T (AT) and activated B (AB) lymphocytes which regulate cytokine and immunoglobulin synthesis respectively [143,144,145,146,147]. When 25(OH)D levels are ~30 ng/mL, it reduces risk of many common cancers [130,131,132,133,134,135,136,137,138,139,140]. It is believed that the local production of 1,25(OH)2D in the breast, colon, prostate, and other cells regulates a variety of genes that control proliferation. Once 1,25(OH)2D completes the task of maintaining normal cellular proliferation and differentiation, it induces the 25-hydroxyvitamin D-24-hydroxylase (24-OHase). The 24-OHase enhances the metabolism of 1,25(OH)2D to calcitroic acid which is biologically inert [7,18]. Thus, the local production of 1,25(OH)2D does not enter the circulation and has no influence on calcium metabolism. The parathyroid glands have 1-OHase activity [45] and the local production of 1,25(OH)2D inhibits the expression and synthesis of PTH [74]. The production of 1,25(OH)2D in the kidney enters the circulation and is able to downregulate renin production in the kidney [148,149] and to stimulate insulin secretion in the β-islet cells of the pancreas [148,150]. Note: This figure is reproduced with permission from [21], Copyright © 2007 Michael F. Holick.
Figure 6
A Schematic representation of the major causes for vitamin D deficiency and potential health consequences. Note: This figure is reproduced with permission from [21], Copyright © 2007 Michael F. Holick.
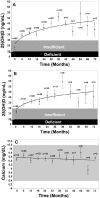
Figure 7
(A) Mean serum 25-hydroxyvitamin D [25(OH)D] levels in all patients: includes patients treated with 50,000 IU vitamin D2 every 2 weeks (maintenance therapy, n = 81), including those patients with vitamin D insufficiency who were initially treated with 8 weeks of 50,000 IU vitamin D2 weekly prior to maintenance therapy (n = 39). Error bars represent standard error of the mean, mean result over 5 years shown. Time 0 is initiation of treatment, results shown as mean values averaged for 6 month intervals. When mean 25(OH)D in each 6 month group was compared to mean initial 25(OH)D, a significant difference was shown with p < 0.001 up until month 43 and p < 0.001 when all remaining values after month 43 were compared to mean initial 25(OH)D. (B) Mean serum 25(OH)D levels in patients receiving maintenance therapy only: Levels for 37 patients who were vitamin D insufficient (25(OH)D levels <30 ng/mL) and 5 patients who were vitamin D sufficient (25(OH)D levels ≥30 ng/mL) who were treated with maintenance therapy of 50,000 IU vitamin D2 every two weeks. Error bars represent standard error of the mean, mean result over 5 years shown. Time 0 is initiation of treatment, results shown as mean values averaged for 6 month intervals. When mean 25(OH)D in each 6 month group were compared to mean initial 25(OH)D, a significant difference was shown with p < 0.001 up until month 37 and p < 0.001 when all remaining values after month 43 were compared to mean initial 25(OH)D. (C) Serum calcium levels: Results for all 81 patients who were treated with 50,000 IU of vitamin D2. Error bars represent standard error of the mean. Time 0 is initiation of treatment, results shown as mean values averaged for 6 month intervals. Normal serum calcium: 8.5–10.2 mg/dL.Note: This figure is reproduced with permission from [242], Copyright © 2009 American Medical Association.
Similar articles
- Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis.
Holick MF. Holick MF. Am J Clin Nutr. 2004 Mar;79(3):362-71. doi: 10.1093/ajcn/79.3.362. Am J Clin Nutr. 2004. PMID: 14985208 Review. - Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare.
Gröber U, Spitz J, Reichrath J, Kisters K, Holick MF. Gröber U, et al. Dermatoendocrinol. 2013 Jun 1;5(3):331-47. doi: 10.4161/derm.26738. Epub 2013 Nov 5. Dermatoendocrinol. 2013. PMID: 24516687 Free PMC article. Review. - Vitamin D history part III: the "modern times"-new questions for orthopaedic practice: deficiency, cell therapy, osteomalacia, fractures, supplementation, infections.
Hernigou P, Sitbon J, Dubory A, Auregan JC. Hernigou P, et al. Int Orthop. 2019 Jul;43(7):1755-1771. doi: 10.1007/s00264-019-04334-w. Epub 2019 Apr 29. Int Orthop. 2019. PMID: 31037319 Review. - Vitamin D: evolutionary, physiological and health perspectives.
Holick MF. Holick MF. Curr Drug Targets. 2011 Jan;12(1):4-18. doi: 10.2174/138945011793591635. Curr Drug Targets. 2011. PMID: 20795941 Review. - Calcium and vitamin D nutrition and bone disease of the elderly.
Gennari C. Gennari C. Public Health Nutr. 2001 Apr;4(2B):547-59. doi: 10.1079/phn2001140. Public Health Nutr. 2001. PMID: 11683549 Review.
Cited by
- Association Between Decreased Serum Vitamin D Level and Dyslipidemia: A Cross-Sectional Study in Southern Taiwan.
Chou SK, Loke SS, Lan C, Sun CF, Huang YH, Huang CF. Chou SK, et al. Int J Gen Med. 2024 Sep 27;17:4369-4376. doi: 10.2147/IJGM.S480241. eCollection 2024. Int J Gen Med. 2024. PMID: 39355338 Free PMC article. - Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery.
Aggeletopoulou I, Kalafateli M, Geramoutsos G, Triantos C. Aggeletopoulou I, et al. Biomolecules. 2024 Aug 30;14(9):1090. doi: 10.3390/biom14091090. Biomolecules. 2024. PMID: 39334856 Free PMC article. Review. - Seasonal variation of total and bioavailable 25-hydroxyvitamin D [25(OH)D] in the healthy adult Slovenian population.
Osredkar J, Vičič V, Hribar M, Benedik E, Siuka D, Jerin A, Čegovnik Primožič U, Fabjan T, Kumer K, Pravst I, Žmitek K. Osredkar J, et al. Acta Biochim Pol. 2024 Sep 11;71:13108. doi: 10.3389/abp.2024.13108. eCollection 2024. Acta Biochim Pol. 2024. PMID: 39323456 Free PMC article. - Role of vitamin D in COVID-19 and other viral infections.
Engin MMN, Özdemir Ö. Engin MMN, et al. World J Virol. 2024 Sep 25;13(3):95349. doi: 10.5501/wjv.v13.i3.95349. World J Virol. 2024. PMID: 39323448 Free PMC article. Review. - The vitamin D status in a Chinese osteogenesis imperfecta population and its correlation with bone metabolic markers and bone density.
Jiang Y, Mei Y, Tian Y, Shen L, Xu S, Zhang H, Zhang Z. Jiang Y, et al. Front Nutr. 2024 Aug 5;11:1390668. doi: 10.3389/fnut.2024.1390668. eCollection 2024. Front Nutr. 2024. PMID: 39161912 Free PMC article.
References
- Holick M. Phylogenetic and Evolutionary Aspects of Vitamin D from Phytoplankton to Humans. In: Pang P.K.T., Schreibman M.P., editors. Verebrate Endocrinology: Fundamentals and Biomedical Implications. Academic Press, Inc.; Orlando, FL, USA: 1989.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical