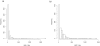Measurement of Plasmodium falciparum transmission intensity using serological cohort data from Indonesian schoolchildren - PubMed (original) (raw)
Measurement of Plasmodium falciparum transmission intensity using serological cohort data from Indonesian schoolchildren
Michael T Bretscher et al. Malar J. 2013.
Abstract
Background: As malaria transmission intensity approaches zero, measuring it becomes progressively more difficult and inefficient because parasite-positive individuals are hard to detect. This situation may arise shortly before achieving local elimination, or during surveillance post-elimination to prevent reintroduction. Antibody responses against the parasite last longer than the infections themselves. This "footprint" of infection may thus be used for assessing transmission intensity. A statistical approach is presented for measuring the seroconversion rate (SCR), a correlate of the force of infection, from individual-level longitudinal data on antibody titres in an area of low Plasmodium falciparum transmission.
Methods: Blood samples were collected from 160 Indonesian schoolchildren every month for six months. Titres of antibodies against AMA-1 and MSP-1(19) antigens of P. falciparum were measured using ELISA. The distribution of antibody titres among seronegative and -positive individuals, respectively, was estimated by comparing the titres from the study data (a mixture of both seropositive and -negative individuals) with titres from a (unexposed) negative control group of Indonesian individuals. Two Markov-Chain models for the transition of individuals between serological states were fitted to individual anti-PfAMA-1 or anti-PfMSP-1 titre time series using Bayesian Markov-Chain-Monte-Carlo (MCMC). This yielded estimates of SCR as well as of the duration of seropositivity.
Results: A posterior median SCR of 0.02 (Pf AMA-1) and 0.09 (PfMSP-1) person(-1) year(-1) was estimated, with credible intervals ranging from 1E-4 to 0.2 person(-1) year(-1). This level of transmission intensity is at the lower range of what can reliably be measured with the present study size. A Bayesian test for seroconversion of an individual between two observations is presented and used to identify the subjects who have most likely experienced an infection. Furthermore, the theoretical limits of measuring transmission intensity, and how these depend on duration and size of a study as well as on transmission intensity itself, is illustrated.
Conclusions: This analysis shows that it is possible to measure SCR's from individual-level longitudinal data on antibody titres. In addition, individual seroconversion events can be identified, which can be useful in assessing interruption of transmission. Analyses of further serological datasets using the present method are required to improve and validate it. This includes measurement of the duration of antibody responses, how it depends on host age or cumulative exposure, or on the particular antigen used.
Figures
Figure 1
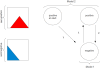
Models for the transitions between serological states. Two models for the transition of individuals from positive (top row) to negative (bottom row) serological states over the course of the study: Model 1 assumes one positive and one negative state, with conversion rate λ and reversion rate ρ. Model 2 treats individuals separately which are already seropositive at the start of the study. Those revert at a rate γ, which may be different from ρ. Titre distributions in positive and negative individuals are schematically indicated on the left.
Figure 2
Distribution of AMA-1 and MSP-2 titres. The titre distribution of anti AMA-1 (a) and anti MSP-1 (b) antibodies in the study population, all survey rounds pooled.
Figure 3
Results of the mixture decomposition. Graphical representation of the estimated probability densities of the titre distributions in seronegative and -positive individuals, respectively, for both AMA-1 (a) and MSP-1 antibodies (b). An average of the PDFs, weighted by relative abundance of positive and negative individuals, approximately yields the PDF of the titre data.
Figure 4
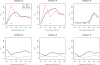
Seroconverting individuals. Those individuals most likely experiencing seroconversion during the study period are displayed in descending order of conversion probability, as determined from pairs of surveys. Only individuals 76, 70 and 78 appear to have converted with near certainty, and do not revert within the study period. Black dots indicate the presence of parasites in blood slides. Since parasitaemic individuals were always treated, those may represent re-infections.
Figure 5
Titre time series. Antibody titre time series of the three individuals which likely seroconverted during the study (red) are shown against the background of the whole study populations; separately for antibodies against AMA-1 (a) and MSP-1 (b).
Figure 6
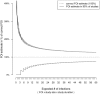
Theoretical limits for measuring transmission intensity in cohort studies. Measuring the force of infection (FOI) in a cohort study by detecting infection events is subject to the theoretical limitations governing count data: under idealizing assumptions the number of infections in a study is Poisson-distributed with expectation equal to FOI x the number of study subjects x study duration. This introduces uncertainty into FOI estimates. An example: in order to measure a FOI with ca. ± 25% accuracy, on average 50 infections need to happen during a study. At a FOI of 0.1, this can be achieved by following 500 individuals for 1 year, or 250 individuals for 2 years, etc.
Similar articles
- Serological markers for monitoring historical changes in malaria transmission intensity in a highly endemic region of Western Kenya, 1994-2009.
Wong J, Hamel MJ, Drakeley CJ, Kariuki S, Shi YP, Lal AA, Nahlen BL, Bloland PB, Lindblade KA, Were V, Otieno K, Otieno P, Odero C, Slutsker L, Vulule JM, Gimnig JE. Wong J, et al. Malar J. 2014 Nov 22;13:451. doi: 10.1186/1475-2875-13-451. Malar J. 2014. PMID: 25416454 Free PMC article. - Seasonal changes in the antibody responses against Plasmodium falciparum merozoite surface antigens in areas of differing malaria endemicity in Indonesia.
Supargiyono S, Bretscher MT, Wijayanti MA, Sutanto I, Nugraheni D, Rozqie R, Kosasih AA, Sulistyawati S, Hawley WA, Lobo NF, Cook J, Drakeley CJ. Supargiyono S, et al. Malar J. 2013 Dec 9;12:444. doi: 10.1186/1475-2875-12-444. Malar J. 2013. PMID: 24321092 Free PMC article. - Using serological measures to monitor changes in malaria transmission in Vanuatu.
Cook J, Reid H, Iavro J, Kuwahata M, Taleo G, Clements A, McCarthy J, Vallely A, Drakeley C. Cook J, et al. Malar J. 2010 Jun 16;9:169. doi: 10.1186/1475-2875-9-169. Malar J. 2010. PMID: 20553604 Free PMC article. - The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis.
Fowkes FJ, Richards JS, Simpson JA, Beeson JG. Fowkes FJ, et al. PLoS Med. 2010 Jan 19;7(1):e1000218. doi: 10.1371/journal.pmed.1000218. PLoS Med. 2010. PMID: 20098724 Free PMC article. Review. - Serology: a robust indicator of malaria transmission intensity?
Corran P, Coleman P, Riley E, Drakeley C. Corran P, et al. Trends Parasitol. 2007 Dec;23(12):575-82. doi: 10.1016/j.pt.2007.08.023. Epub 2007 Nov 7. Trends Parasitol. 2007. PMID: 17988945 Review.
Cited by
- Anti-malarial seroprevalence assessment during an elimination programme in Chabahar District, south-eastern Iran.
Zakeri S, van den Hoogen LL, Mehrizi AA, Karimi F, Raeisi A, Drakeley C. Zakeri S, et al. Malar J. 2016 Jul 22;15(1):382. doi: 10.1186/s12936-016-1432-1. Malar J. 2016. PMID: 27448606 Free PMC article. - Development of new malaria diagnostics: matching performance and need.
Bell D, Fleurent AE, Hegg MC, Boomgard JD, McConnico CC. Bell D, et al. Malar J. 2016 Aug 11;15(1):406. doi: 10.1186/s12936-016-1454-8. Malar J. 2016. PMID: 27515426 Free PMC article. - Analysis of antibody profiles in symptomatic malaria in three sentinel sites of Ivory Coast by using multiplex, fluorescent, magnetic, bead-based serological assay (MAGPIX™).
Koffi D, Touré AO, Varela ML, Vigan-Womas I, Béourou S, Brou S, Ehouman MF, Gnamien L, Richard V, Djaman JA, Perraut R. Koffi D, et al. Malar J. 2015 Dec 21;14:509. doi: 10.1186/s12936-015-1043-2. Malar J. 2015. PMID: 26692284 Free PMC article. - Defining Seropositivity Thresholds for Use in Trachoma Elimination Studies.
Migchelsen SJ, Martin DL, Southisombath K, Turyaguma P, Heggen A, Rubangakene PP, Joof H, Makalo P, Cooley G, Gwyn S, Solomon AW, Holland MJ, Courtright P, Willis R, Alexander ND, Mabey DC, Roberts CH. Migchelsen SJ, et al. PLoS Negl Trop Dis. 2017 Jan 18;11(1):e0005230. doi: 10.1371/journal.pntd.0005230. eCollection 2017 Jan. PLoS Negl Trop Dis. 2017. PMID: 28099433 Free PMC article. - Multiple comparisons analysis of serological data from an area of low Plasmodium falciparum transmission.
Rogier E, Wiegand R, Moss D, Priest J, Angov E, Dutta S, Journel I, Jean SE, Mace K, Chang M, Lemoine JF, Udhayakumar V, Barnwell JW. Rogier E, et al. Malar J. 2015 Nov 4;14:436. doi: 10.1186/s12936-015-0955-1. Malar J. 2015. PMID: 26537125 Free PMC article.
References
- Draper CC, Voller A, Carpenter RG. The epidemiologic interpretation of serologic data in malaria. Am J Trop Med Hyg. 1972;21:696–703. - PubMed
- Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SLR, Carneiro I, Malima R, Lusingu J, Manjurano A, Nkya WMM, Lemnge MM, Cox J, Reyburn H, Riley EM. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials