Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes - PubMed (original) (raw)
Review
Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes
Andrea Cerutti et al. Nat Rev Immunol. 2013 Feb.
Abstract
Protective responses to microorganisms involve the nonspecific but rapid defence mechanisms of the innate immune system, followed by the specific but slow defence mechanisms of the adaptive immune system. Located as sentinels at the interface between the circulation and lymphoid tissue, splenic marginal zone B cells rapidly respond to blood-borne antigens by adopting 'crossover' defensive strategies that blur the conventional boundaries of innate and adaptive immunity. This Review discusses how marginal zone B cells function as innate-like lymphocytes that mount rapid antibody responses to both T cell-dependent and T cell-independent antigens. These responses require the integration of activation signals from germline-encoded and somatically recombined receptors for microorganisms with helper signals from effector cells of the innate and adaptive immune systems.
Conflict of interest statement
Competing interests statement
The authors declare no competing financial interests.
Figures
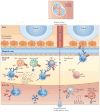
Figure 1. T cell-independent responses by mouse MZ B cells
T cell-independent (Tl) antigens are captured by metallophilic macrophages and marginal zone (MZ) macrophages after entering the splenic MZ via the marginal sinus. Alternatively, Tl antigens are captured by dendritic cells (DCs) and neutrophils in the circulation. Innate response activator (IRA) B cells may enhance the survival and activation of these antigen-capturing cells by releasing granulocyte–macrophage colony-stimulating factor (GM-CSF). Antigen-sampling cells stimulate MZB cells via the B cell receptor (BCR), Toll-like receptors (TLRs) and transmembrane activator and CAML interactor (TACI). TACI delivers signals that induce class-switch recombination and antibody production after ligating B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL), which are released by antigen-capturing cells in response to microbial TLR ligands. Antigen-capturing cells, including red pulp DCs and macrophages, also secrete interleukin-6 (IL-6), IL-10, type I interferons (IFNs) and CXC-chemokine ligand 10 (CXCL10), which cooperate with BAFF and APRIL to promote the differentiation and survival of plasmablasts secreting IgM or class-switched IgG. Arrows indicate the path followed by antigens through the spleen. PALS, periarteriolar lymphoid sheath; PRR, pattern-recognition receptor.
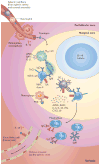
Figure 2. T cell-independent responses by human MZ B cells
T cell-independent (Tl) antigens are thought to enter the marginal zone (MZ) through the perifollicular zone. Once in the MZ, they may be captured by neutrophil extracellular trap (NET)-like structures emanating from B cell-helper neutrophils (NBH cells). These cells may differentiate from circulating neutrophils as a result of the production of interleukin-10 (IL-10) by perifollicular sinus-lining cells and macrophages in response to microbial Toll-like receptor (TLR) ligands. Antigen capture may also involve reticular cells, macrophages, sinus-lining cells and dendritic cells (DCs). In addition to making Tl antigens available to the B cell receptor (BCR) and TLRs on MZ B cells, antigen-capturing cells release B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL), which engage transmembrane activator and CAML interactor (TACI) on MZ B cells. NBH cells also release IL-21, thereby inducing class-switch recombination, somatic hypermutation and antibody production in MZ B cells. The generation of plasmablasts secreting IgM or class-switched IgG and IgA involves the production of IL-6, IL-10, IL-21 and CXC-chemokine ligand 10 (CXCL10) by antigen-capturing cells. Arrows indicate the putative path followed by antigens through the spleen. IFN, interferon; PALS, periarteriolar lymphoid sheath; PRR, pattern-recognition receptor.
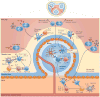
Figure 3. T cell-dependent responses by mouse MZ B cells
In the extrafollicular T cell-dependent (TD) pathway, marginal zone (MZ) dendritic cells (DCs) expressing DC inhibitory receptor 2 (DCIR2) capture protein antigens and present them to naive CD4+ T cells, which subsequently differentiate into T helper 2 (TH2) cells expressing CD40 ligand (CD40L) and interleukin-4 (IL-4). DCIR2+ DCs also expose antigens to MZ B cells, and signals from the B cell receptor (BCR) convert MZB cells into antigen-presenting cells. By establishing cognate interactions with TH2 cells, activated MZ B cells rapidly produce low-affinity IgGl but not IgM. ln an alternative pathway, lipid-reactive MZ B cells activate invariant natural killer T (iNKT) cells by presenting CDld-loaded glycolipids. The expression of CD40L, IL-4 and interferon-γ(IFNγ) by activated iNKT cells induces MZ B cells to differentiate into plasma blasts that secrete low-affinity IgM or IgG. In the follicular TD pathway, MZB cells downregulate receptors for sphin-gosine-1-phosphate (SIP) after capturing complement-decorated protein antigens via CD21 and CD3B. The interruption of MZ-retaining signals from SIP receptors stimulates MZ B cells to enter the follicle in response to CXC-chemokine receptor 5 (CXCRB) signals. In the follicle, MZ B cells deposit antigens on follicular dendritic cells (FDCs) and thereafter upregulate SI P receptor expression to exit the follicle and return to the MZ. Protein antigens displayed by FDCs enhance TD antibody responses by promoting the selection of follicular germinal centre (GC) B cells expressing a high-affinity BCR. This selection also involves cognate interactions between GC B cells and T follicular helper (TFH) cells expressing CD40L and IL-21. Ultimately, selected GC B cells differentiate into long-lived memory B cells or plasma cells that produce high-affinity IgG. Alternatively, MZ B cells capture protein antigens with or without the help of metallophilic macrophages and migrate to the border between the periarteriolar lymphoid sheath (PALS) and the follicle to initiate a GC reaction after establishing a cognate interaction with TFH cells expressing CD40L, IL-4 or interferon-γ(IFNγ). These TFH cells probably emerge from the priming of naive CD4+T cells by either MZ B cells or DCs expressing DEC205. A subset of these TFH cells can also promote the generation of low-affinity extrafollicular IgM and IgG plasmablasts. CXCL13, CXC-chemokine ligand 13; TCR. T cell receptor; TLR, Toll-like receptor.
Similar articles
- The soluble pattern recognition receptor PTX3 links humoral innate and adaptive immune responses by helping marginal zone B cells.
Chorny A, Casas-Recasens S, Sintes J, Shan M, Polentarutti N, García-Escudero R, Walland AC, Yeiser JR, Cassis L, Carrillo J, Puga I, Cunha C, Bastos H, Rodrigues F, Lacerda JF, Morais A, Dieguez-Gonzalez R, Heeger PS, Salvatori G, Carvalho A, Garcia-Sastre A, Blander JM, Mantovani A, Garlanda C, Cerutti A. Chorny A, et al. J Exp Med. 2016 Sep 19;213(10):2167-85. doi: 10.1084/jem.20150282. Epub 2016 Sep 12. J Exp Med. 2016. PMID: 27621420 Free PMC article. - New helping friends for B cells.
Cerutti A, Puga I, Cols M. Cerutti A, et al. Eur J Immunol. 2012 Aug;42(8):1956-68. doi: 10.1002/eji.201242594. Eur J Immunol. 2012. PMID: 22865046 Free PMC article. Review. - Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells.
Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L, Barra CM, Comerma L, Chudnovskiy A, Gentile M, Llige D, Cols M, Serrano S, Aróstegui JI, Juan M, Yagüe J, Merad M, Fagarasan S, Cerutti A. Magri G, et al. Nat Immunol. 2014 Apr;15(4):354-364. doi: 10.1038/ni.2830. Epub 2014 Feb 23. Nat Immunol. 2014. PMID: 24562309 Free PMC article. - T-Independent immune response: new aspects of B cell biology.
Fagarasan S, Honjo T. Fagarasan S, et al. Science. 2000 Oct 6;290(5489):89-92. doi: 10.1126/science.290.5489.89. Science. 2000. PMID: 11021805 Review. - Regulation of frontline antibody responses by innate immune signals.
Chorny A, Puga I, Cerutti A. Chorny A, et al. Immunol Res. 2012 Dec;54(1-3):4-13. doi: 10.1007/s12026-012-8307-5. Immunol Res. 2012. PMID: 22477522 Free PMC article. Review.
Cited by
- Frequencies or Absolute Numbers? Cluster Analysis of Frequencies and Absolute Numbers of B-Cell Subsets in Dialysis Patients Who Are Candidates for Kidney Transplantation Reveals Different Profiles.
Fouza A, Fylaktou A, Tagkouta A, Daoudaki M, Vagiotas L, Kasimatis E, Xochelli A, Nikolaidou V, Katsanos G, Tsoulfas G, Skoura L, Papagianni A, Antoniadis N. Fouza A, et al. J Clin Med. 2024 Oct 28;13(21):6454. doi: 10.3390/jcm13216454. J Clin Med. 2024. PMID: 39518592 Free PMC article. - Phosphatidylserine phospholipase A1 enables GPR34-dependent immune cell accumulation in the peritoneal cavity.
Tam H, Xu Y, An J, Schöneberg T, Schulz A, Muppidi JR, Cyster JG. Tam H, et al. J Exp Med. 2024 Nov 4;221(11):e20240992. doi: 10.1084/jem.20240992. Epub 2024 Oct 16. J Exp Med. 2024. PMID: 39412501 Free PMC article. - Germinal center B-cell subgroups in the tumor microenvironment cannot be overlooked: Their involvement in prognosis, immunotherapy response, and treatment resistance in head and neck squamous carcinoma.
Lin L, Zou J, Pei S, Huang W, Zhang Y, Zhao Z, Ding Y, Xiao C. Lin L, et al. Heliyon. 2024 Sep 11;10(19):e37726. doi: 10.1016/j.heliyon.2024.e37726. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39391510 Free PMC article. - The Pathology of the Brain Eating Amoeba Naegleria fowleri.
Borkens Y. Borkens Y. Indian J Microbiol. 2024 Sep;64(3):1384-1394. doi: 10.1007/s12088-024-01218-5. Epub 2024 Mar 6. Indian J Microbiol. 2024. PMID: 39282207 Free PMC article. - Rack1 regulates B-cell development and function by binding to and stabilizing the transcription factor Pax5.
Zhang X, Ma C, Lu Y, Wang J, Yun H, Jiang H, Wu M, Feng X, Gai W, Xu G, Deng H, Feng J, Liu W, Shi T, Cheng Q, Zhang J. Zhang X, et al. Cell Mol Immunol. 2024 Nov;21(11):1282-1295. doi: 10.1038/s41423-024-01213-2. Epub 2024 Sep 10. Cell Mol Immunol. 2024. PMID: 39256480
References
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. - PubMed
- Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. - PubMed
- Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nature Rev Immunol. 2001;1:177–186. - PubMed
- Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. - PubMed
- Martin F, Kearney JF. Marginal-zone B cells. Nature Rev Immunol. 2002;2:323–335. References 3 and 5 describe the function, ontogeny and reactivity of MZ B cells. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI95613/AI/NIAID NIH HHS/United States
- AI61093/AI/NIAID NIH HHS/United States
- U01 AI095613/AI/NIAID NIH HHS/United States
- AI96187/AI/NIAID NIH HHS/United States
- AI074378/AI/NIAID NIH HHS/United States
- P01 AI061093/AI/NIAID NIH HHS/United States
- R01 AI074378/AI/NIAID NIH HHS/United States
- U19 AI096187/AI/NIAID NIH HHS/United States
- R01 AI057653/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources