Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension - PubMed (original) (raw)
. 2013 Feb;123(2):657-65.
doi: 10.1172/JCI61110. Epub 2013 Jan 25.
Dominique Loffing-Cueni, Pierrette Hausel, Anne Debonneville, Sumedha Ram Malsure, Nicole Fowler-Jaeger, Natasha A Boase, Romain Perrier, Marc Maillard, Baoli Yang, John B Stokes, Robert Koesters, Sharad Kumar, Edith Hummler, Johannes Loffing, Olivier Staub
Affiliations
- PMID: 23348737
- PMCID: PMC3561795
- DOI: 10.1172/JCI61110
Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension
Caroline Ronzaud et al. J Clin Invest. 2013 Feb.
Abstract
The E3 ubiquitin ligase NEDD4-2 (encoded by the Nedd4L gene) regulates the amiloride-sensitive epithelial Na+ channel (ENaC/SCNN1) to mediate Na+ homeostasis. Mutations in the human β/γENaC subunits that block NEDD4-2 binding or constitutive ablation of exons 6-8 of Nedd4L in mice both result in salt-sensitive hypertension and elevated ENaC activity (Liddle syndrome). To determine the role of renal tubular NEDD4-2 in adult mice, we generated tetracycline-inducible, nephron-specific Nedd4L KO mice. Under standard and high-Na+ diets, conditional KO mice displayed decreased plasma aldosterone but normal Na+/K+ balance. Under a high-Na+ diet, KO mice exhibited hypercalciuria and increased blood pressure, which were reversed by thiazide treatment. Protein expression of βENaC, γENaC, the renal outer medullary K+ channel (ROMK), and total and phosphorylated thiazide-sensitive Na+Cl- cotransporter (NCC) levels were increased in KO kidneys. Unexpectedly, Scnn1a mRNA, which encodes the αENaC subunit, was reduced and proteolytic cleavage of αENaC decreased. Taken together, these results demonstrate that loss of NEDD4-2 in adult renal tubules causes a new form of mild, salt-sensitive hypertension without hyperkalemia that is characterized by upregulation of NCC, elevation of β/γENaC, but not αENaC, and a normal Na+/K+ balance maintained by downregulation of ENaC activity and upregulation of ROMK.
Figures
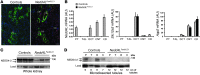
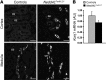
Figure 1. Generation of inducible renal tubule–specific Nedd4L KO mice.
Nedd4Lflox/flox/Pax8-rTA/LC1 KO mice and control littermates (Nedd4LPax8 or Nedd4LLC1) were obtained and treated with doxycycline as described previously (24) to induce Nedd4L ablation in renal tubular cells. (A) Immunofluorescence for CRE and calbindin CaBP28 on kidney sections from control (left panel) and induced Nedd4LPax8/LC1 KO (right panel). CRE recombinase is expressed in all renal tubules in induced KO mice, including CaBP28-positive DCT/CNT cells (CRE: red; CaBP28: green; DAPI: blue). Scale bars: ∼50 μm. C, CNT; D, DCT. (B) Quantitative real-time PCR analysis for Nedd4L, Slc12a3 (encoding NCC), and aquaporin 2 (Aqp2) mRNA on microdissected renal tubules normalized to Gapdh: Nedd4L mRNA is not detected in Nedd4LPax8/LC1 KO, and Slc12a3 mRNA levels are unchanged (n = 4 per group, 8 days of high-Na+ diet). Slc12a3 was used as DCT marker and Aqp2 as CNT/CD marker. (C and D) Analysis of NEDD4-2 protein expression by Western blot in whole kidney lysates (C) and microdissected tubules (D). The lower NEDD4-2 expression observed in KO whole-kidney lysates is absent in microdissected tubules (n = 3 mice per genotype). Load: unspecific band used as loading control. C, CNT/CD; P, PT; T, TAL.
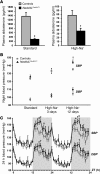
Figure 2. Inducible Nedd4LPax8/LC1 KO mice show decreased plasma aldosterone and salt-sensitive blood pressure increase.
(A) Plasma aldosterone levels were decreased in induced Nedd4LPax8/LC1 KO (n = 7–10) compared with controls (n = 6–9) under both standard and high-Na+ diets. (B) Graphs represent SBP and DBP 12-hour night averages measured by telemetry in controls (n = 8) and Nedd4LPax8/LC1 KO (n = 6) under standard diet and after 3 and 12 days of high-Na+ diet. (C) SBP and DBP 24-hour profiles (day, white zone; night, gray zone) in controls (gray curve) and Nedd4LPax8/LC1 KO (black curve) after 12 days of high-Na+ diet. ZT, Zeitgeber time (ZT0 or ZT24, light on; ZT12, light off). *P < 0.05, †P < 0.01, ‡P < 0.005, KO versus controls.
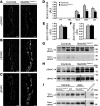
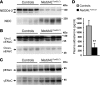
Figure 3. Nedd4LPax8/LC1 KO mice have increased β- and γENaC abundance, but not αENaC.
(A–C) Immunostaining for α- (A), β- (B), and γENaC (C) on kidney cryosections of control and KO mice under high-Na+ diet. Cytoplasmic β- and γENaC abundance is increased in CD of KO, whereas αENaC is decreased. Scale bars: ∼20 μm. (D) TaqMan analysis of Scnn1a mRNA on microdissected renal tubules shows downregulation in KO (n = 4 per group). (E and F) TaqMan analysis for Scnn1b (E) and Scnn1g (F) mRNA on whole kidney (n = 6 per genotype). mRNA expression was normalized to Gapdh mRNA levels and expressed relative to control values. (G and H) Representative Western blots for α-, β-, and γENaC: the cleaved 30-kDa αENaC band was decreased in Nedd4LPax8/LC1 KO mice (G), whereas β- and γENaC expression were elevated (H). For γENaC, lanes were run on the same gel but were noncontiguous, as indicated by the vertical black line. (I) Aldosterone infusion in Nedd4LPax8/LC1 KO mice results in increased αENaC protein (uncleaved and cleaved form) expression, suggesting that the reduced expression observed in the KO is due to the decreased plasma aldosterone. Kidney extract from Scnn1a KO mice was used as negative control. Lanes that were run on the same gel but were noncontiguous are indicated with the vertical black lines. *P < 0.05, KO versus controls. Ctrl, control mice.
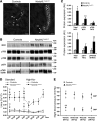
Figure 4. NCC is overactivated in Nedd4LPax8/LC1 KO mice.
(A) Immunofluorescence for NCC on kidney sections of control and KO mice under high-Na+ diet. NCC expression is increased in DCT of KO. Scale bars: ∼20 μm. (B) Western blot analysis for total NCC and phosphorylated pT53-, pT58-, pS71-, and pT43/53/58-NCC. A representative blot on 5 controls and 5 KO is shown. For pS71-NCC and pT43/53/58-NCC, lanes that were run on the same gel but noncontiguous are indicated with a vertical black line. (C) Graphs show quantification of Western blots for phosphorylated NCC and the corresponding total NCC from 2 independent experiments (pT53-NCC and pT58-NCC: n = 5 per genotype; pS71-NCC and p43/53/58-NCC: n = 11 per genotype). Protein expression was normalized to the amount of β-actin or β-tubulin and expressed relative to control values. (D) Urine Ca2+ measurement in control and KO mice under high-Na+ diet (10 days). Nedd4LPax8/LC1 KO are hypercalciuric as shown by the 3-fold increased urine Ca2+/creatinine (Cr) ratio, which can be corrected by thiazide (n = 4 per group). Vh, vehicle; Tz, thiazide. (E) Plot showing telemetric measurement on controls versus KO under high-Na+ diet. The increased SBP and DBP observed in KO after 5 weeks of high-Na+ diet can be prevented by thiazide, but not by amiloride (n = 4 per group). *P < 0.05, **P < 0.01, KO versus controls. †P < 0.05, ††P < 0.01, thiazide versus vehicle.
Figure 5. Nedd4LPax8/LC1 KO mice have increased ROMK abundance.
(A) Immunofluorescence for ROMK on kidney sections from control and KO mice fed with high-Na+ diet (10 days) shows increase in the channel in DCT2 (D2), CNT (CN), CD and TAL (T) in KO. Scale bars: ∼40 μm. (B) TaqMan analysis showed that Kcnj1 mRNA levels are not changed between controls and KO mice on whole kidney.
Figure 6. Nedd4L ablation in renal tubules during embryonic development results in phenotype similar to that seen when the deletion occurs at adult age.
Mice were treated with doxycycline during embryonic development to induce Nedd4L ablation early on. (A–C) Western blot analysis on 5-week-old control and KO mice showed increased NCC (A), β-, and γENaC (C) expression, and decreased cleaved αENaC under high-Na+ diet (10 days) (B). (D) Plasma aldosterone levels were decreased in KO (n = 7) compared with control mice (n = 7) under standard diet. *P < 0.05, KO versus controls.
Comment in
- Ubiquitylation and the pathogenesis of hypertension.
Ellison DH. Ellison DH. J Clin Invest. 2013 Feb;123(2):546-8. doi: 10.1172/JCI66882. Epub 2013 Jan 25. J Clin Invest. 2013. PMID: 23348731 Free PMC article.
Similar articles
- Deletion of renal Nedd4-2 abolishes the effect of high sodium intake (HS) on Kir4.1, ENaC, and NCC and causes hypokalemia during high HS.
Zhang DD, Duan XP, Xiao Y, Wu P, Gao ZX, Wang WH, Lin DH. Zhang DD, et al. Am J Physiol Renal Physiol. 2021 May 1;320(5):F883-F896. doi: 10.1152/ajprenal.00555.2020. Epub 2021 Apr 5. Am J Physiol Renal Physiol. 2021. PMID: 33818128 Free PMC article. - Ubiquitylation and the pathogenesis of hypertension.
Ellison DH. Ellison DH. J Clin Invest. 2013 Feb;123(2):546-8. doi: 10.1172/JCI66882. Epub 2013 Jan 25. J Clin Invest. 2013. PMID: 23348731 Free PMC article. - ROMK channels are inhibited in the aldosterone-sensitive distal nephron of renal tubule Nedd4-2-deficient mice.
Zhang DD, Zheng JY, Duan XP, Lin DH, Wang WH. Zhang DD, et al. Am J Physiol Renal Physiol. 2022 Jan 1;322(1):F55-F67. doi: 10.1152/ajprenal.00306.2021. Epub 2021 Nov 29. Am J Physiol Renal Physiol. 2022. PMID: 34843409 Free PMC article. - NEDD4-2 and salt-sensitive hypertension.
Rizzo F, Staub O. Rizzo F, et al. Curr Opin Nephrol Hypertens. 2015 Mar;24(2):111-6. doi: 10.1097/MNH.0000000000000097. Curr Opin Nephrol Hypertens. 2015. PMID: 25602517 Review. - NEDD4-2 (NEDD4L): the ubiquitin ligase for multiple membrane proteins.
Goel P, Manning JA, Kumar S. Goel P, et al. Gene. 2015 Feb 15;557(1):1-10. doi: 10.1016/j.gene.2014.11.051. Epub 2014 Nov 26. Gene. 2015. PMID: 25433090 Free PMC article. Review.
Cited by
- Urine Cell Transcriptomes Implicate Specific Renal Inflammatory Pathways Associated With Difficult-to-Control Hypertension.
Umanath K, She R, Hassett C, Adrianto I, Levin AM, Savickas G, Yee J, Ortiz P. Umanath K, et al. J Am Heart Assoc. 2023 Mar 21;12(6):e026242. doi: 10.1161/JAHA.122.026242. Epub 2023 Mar 9. J Am Heart Assoc. 2023. PMID: 36892045 Free PMC article. - Ubiquitination of NKCC2 by the cullin-RING E3 ubiquitin ligase family in the thick ascending limb of the loop of Henle.
Ares GR. Ares GR. Am J Physiol Renal Physiol. 2023 Mar 1;324(3):F315-F328. doi: 10.1152/ajprenal.00079.2022. Epub 2023 Feb 2. Am J Physiol Renal Physiol. 2023. PMID: 36727946 Free PMC article. - Molecular Mechanisms of Na-Cl Cotransporter in Relation to Hypertension in Chronic Kidney Disease.
Liang L, Shimosawa T. Liang L, et al. Int J Mol Sci. 2022 Dec 23;24(1):286. doi: 10.3390/ijms24010286. Int J Mol Sci. 2022. PMID: 36613730 Free PMC article. Review. - The serine-threonine kinase PIM3 is an aldosterone-regulated protein in the distal nephron.
Spirli A, Cheval L, Debonneville A, Penton D, Ronzaud C, Maillard M, Doucet A, Loffing J, Staub O. Spirli A, et al. Physiol Rep. 2019 Aug;7(15):e14177. doi: 10.14814/phy2.14177. Physiol Rep. 2019. PMID: 31397090 Free PMC article. - The sodium chloride cotransporter SLC12A3: new roles in sodium, potassium, and blood pressure regulation.
Moes AD, van der Lubbe N, Zietse R, Loffing J, Hoorn EJ. Moes AD, et al. Pflugers Arch. 2014 Jan;466(1):107-18. doi: 10.1007/s00424-013-1407-9. Epub 2013 Dec 6. Pflugers Arch. 2014. PMID: 24310820 Review.
References
- Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000;80(1):277–313. - PubMed
- Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol. 1998;274(5 pt 2):F817–F833. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials