Mammalian Clusterin associated protein 1 is an evolutionarily conserved protein required for ciliogenesis - PubMed (original) (raw)
Mammalian Clusterin associated protein 1 is an evolutionarily conserved protein required for ciliogenesis
Raymond C Pasek et al. Cilia. 2012.
Abstract
Background: Clusterin associated protein 1 (CLUAP1) was initially characterized as a protein that interacts with clusterin, and whose gene is frequently upregulated in colon cancer. Although the consequences of these observations remain unclear, research of CLUAP1 homologs in C. elegans and zebrafish indicates that it is needed for cilia assembly and maintenance in these models. To begin evaluating whether Cluap1 has an evolutionarily conserved role in cilia in mammalian systems and to explore the association of Cluap1 with disease pathogenesis and developmental abnormalities, we generated Cluap1 mutant mice.
Methods: Cluap1 mutant embryos were generated and examined for gross morphological and anatomical defects using light microscopy. Reverse transcription PCR, β-galactosidase staining assays, and immunofluorescence analysis were used to determine the expression of the gene and localization of the protein in vivo and in cultured cell lines. We also used immunofluorescence analysis and qRT-PCR to examine defects in the Sonic hedgehog signaling pathway in mutant embryos.
Results: Cluap1 mutant embryos die in mid-gestation, indicating that it is necessary for proper development. Mutant phenotypes include a failure of embryonic turning, an enlarged pericardial sac, and defects in neural tube development. Consistent with the diverse phenotypes, Cluap1 is widely expressed. Furthermore, the Cluap1 protein localizes to primary cilia, and mutant embryos were found to lack cilia at embryonic day 9.5. The phenotypes observed in Cluap1 mutant mice are indicative of defects in Sonic hedgehog signaling. This was confirmed by analyzing hedgehog signaling activity in Cluap1 mutants, which revealed that the pathway is repressed.
Conclusions: These data indicate that the function of Cluap1 is evolutionarily conserved with regard to ciliogenesis. Further, the results implicate mammalian Cluap1 as a key regulator of hedgehog signaling and as an intraflagellar transport B complex protein. Future studies on mammalian Cluap1 utilizing this mouse model may provide insights into the role for Cluap1 in intraflagellar transport and the association with colon cancer and cystic kidney disorders.
Figures
Figure 1
Clusterin associated protein 1 (Cluap1) knockout mice are embryonic lethal. (A) Schematic of the wild-type Cluap1 allele (Cluap1WT) and the Cluap1 knockout allele (Cluap1KO). The relative position of the β-galactosidase cassette is indicated by the blue box. (B) PCR genotyping of Cluap1WT, Cluap1Het, and Cluap1KO embryos. (C) At E9.5, Cluap1KO embryos are runted, have enlarged pericardial sacs (arrow), and fail to turn properly (asterisk). (D) RT-PCR gel showing the expression of Cluap1 transcript in both Cluap1WT and Cluap1Het embryos and the absence in Cluap1KO embryos. Actin served as a positive template control in all samples. Reactions treated with reverse transcriptase (“+”) are alongside negative RT control samples (“- “). (E) Loss of the wild-type Cluap1 protein in Cluap1KO embryos was determined by Western blot. Actin was used as a loading control.
Figure 2
Cluap1 is expressed in ciliated cells with a wide tissue distribution. (A) RT-PCR gel showing expression of Cluap1 in the indicated tissues; Sk. Muscle, skeletal muscle. Actin is used as a positive control. Reactions treated with reverse transcriptase (“+”) are alongside negative RT control samples (“- ”). (B) β-Galactosidase staining assay showing Cluap1 expression in Cluap1Het tissue in the ventricles of the heart, cortex of the kidney, lung tissue, and whole E9.5 embryo. Cluap1WT control tissue samples. Heart and kidney sections were counterstained in nuclear fast red. Scale bars are 10 μm in heart sections, 30 μm in kidney sections, and 1,000 μm for whole lung tissues and embryos.
Figure 3
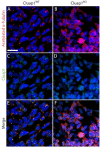
Cluap1 localizes to primary cilia in vitro. Antibody against acetylated α-tubulin (red) and Cluap1 (green) label primary cilia (arrows) in (A-C) NIH3T3 cells (scale bars are 14 μm). (D-F) 176-6C collecting duct epithelium (scale bars are 21 μm) and (G-I) IMCD3 cells (scale bars are 20 μm). Arrows indicate primary cilium. Nuclei are stained blue with Hoechst.
Figure 4
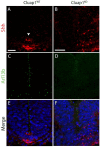
Cluap1 KO embryos fail to form primary cilia. (A,C,E) Cluap1WT E9.5 embryos were immunolabeled for the cilia marker acetylated α-tubulin (red) and Cluap1 (green) in the lateral plate mesenchyme of Cluap1WT embryos. (B,D,F) Cluap1KO embryos show a total loss of cilia in the same region. Hoechst nuclear stain in blue. Scale bar is 31.5 μm.
Figure 5
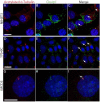
Cluap1 KO embryos have defects in floorplate induction. (A,C,E) Cluap1WT E9.5 embryos stained for Arl13b (green) show cilia in the neural tube and surrounding tissue. Staining for Sonic hedgehog ligand (red) shows a Shh immunopositive floorplate. (B,D,F) Cluap1KO embryos show an absence of cilia as indicated by the lack of Arl13b staining. Note the lack of a clearly defined Shh immunopositive floorplate. Hoechst nuclear stain in blue. Scale bars are 21 μm.
Figure 6
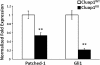
Cluap1 KO embryos have downregulated expression of Patched-1 and Gli1. Real-time PCR results for the expression of Patched-1 and Gli1 in E9.5 Cluap1WT and Cluap1KO embryos demonstrate a significant decrease in expression of both Patched-1 and Gli1. Expression levels are relative to control peptidylprolyl isomerase A (PPIA). Bars represent mean fold expression, and error bars are ± SEM. Asterisks represent significant difference from control (**P < 0.01, Student’s _t_-test).
Similar articles
- Cluap1 is essential for ciliogenesis and photoreceptor maintenance in the vertebrate eye.
Lee C, Wallingford JB, Gross JM. Lee C, et al. Invest Ophthalmol Vis Sci. 2014 Jun 26;55(7):4585-92. doi: 10.1167/iovs.14-14888. Invest Ophthalmol Vis Sci. 2014. PMID: 24970261 Free PMC article. - Cluap1 localizes preferentially to the base and tip of cilia and is required for ciliogenesis in the mouse embryo.
Botilde Y, Yoshiba S, Shinohara K, Hasegawa T, Nishimura H, Shiratori H, Hamada H. Botilde Y, et al. Dev Biol. 2013 Sep 1;381(1):203-12. doi: 10.1016/j.ydbio.2013.05.024. Epub 2013 Jun 4. Dev Biol. 2013. PMID: 23742838 - Mutations in Traf3ip1 reveal defects in ciliogenesis, embryonic development, and altered cell size regulation.
Berbari NF, Kin NW, Sharma N, Michaud EJ, Kesterson RA, Yoder BK. Berbari NF, et al. Dev Biol. 2011 Dec 1;360(1):66-76. doi: 10.1016/j.ydbio.2011.09.001. Epub 2011 Sep 16. Dev Biol. 2011. PMID: 21945076 Free PMC article. - Cilia and developmental signaling.
Eggenschwiler JT, Anderson KV. Eggenschwiler JT, et al. Annu Rev Cell Dev Biol. 2007;23:345-73. doi: 10.1146/annurev.cellbio.23.090506.123249. Annu Rev Cell Dev Biol. 2007. PMID: 17506691 Free PMC article. Review. - Rab23 and developmental disorders.
Hor CHH, Tang BL, Goh ELK. Hor CHH, et al. Rev Neurosci. 2018 Nov 27;29(8):849-860. doi: 10.1515/revneuro-2017-0110. Rev Neurosci. 2018. PMID: 29727300 Review.
Cited by
- Assembly and stability of IFT-B complex and its function in BBSome trafficking.
Wang J, Zhu X, Wang Z, Li X, Tao H, Pan J. Wang J, et al. iScience. 2022 Nov 4;25(12):105493. doi: 10.1016/j.isci.2022.105493. eCollection 2022 Dec 22. iScience. 2022. PMID: 36411782 Free PMC article. - Mosaic origin of the eukaryotic kinetochore.
Tromer EC, van Hooff JJE, Kops GJPL, Snel B. Tromer EC, et al. Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):12873-12882. doi: 10.1073/pnas.1821945116. Epub 2019 May 24. Proc Natl Acad Sci U S A. 2019. PMID: 31127038 Free PMC article. - Genetic variants are identified to increase risk of COVID-19 related mortality from UK Biobank data.
Hu J, Li C, Wang S, Li T, Zhang H. Hu J, et al. Hum Genomics. 2021 Feb 3;15(1):10. doi: 10.1186/s40246-021-00306-7. Hum Genomics. 2021. PMID: 33536081 Free PMC article. - An experimental genetically attenuated live vaccine to prevent transmission of Toxoplasma gondii by cats.
Ramakrishnan C, Maier S, Walker RA, Rehrauer H, Joekel DE, Winiger RR, Basso WU, Grigg ME, Hehl AB, Deplazes P, Smith NC. Ramakrishnan C, et al. Sci Rep. 2019 Feb 6;9(1):1474. doi: 10.1038/s41598-018-37671-8. Sci Rep. 2019. PMID: 30728393 Free PMC article. - Cluap1 is essential for ciliogenesis and photoreceptor maintenance in the vertebrate eye.
Lee C, Wallingford JB, Gross JM. Lee C, et al. Invest Ophthalmol Vis Sci. 2014 Jun 26;55(7):4585-92. doi: 10.1167/iovs.14-14888. Invest Ophthalmol Vis Sci. 2014. PMID: 24970261 Free PMC article.
References
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. - PubMed
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials