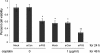Silencing of mutant p53 by siRNA induces cell cycle arrest and apoptosis in human bladder cancer cells - PubMed (original) (raw)
Silencing of mutant p53 by siRNA induces cell cycle arrest and apoptosis in human bladder cancer cells
Hai-Bin Zhu et al. World J Surg Oncol. 2013.
Abstract
Background: p53 is the most frequently mutated tumor-suppressor gene in human cancers. It has been reported that mutations in p53 result not only in the loss of its ability as a tumor suppressor, but also in the gain of novel cancer-related functions that contribute to oncogenesis. The present study evaluated the potential of silencing of mutant p53 by small interfering RNA in the treatment of bladder cancer cells in vitro.
Methods: We used the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to assess cell viability and flow cytometry to detect cell cycle alterations and apoptosis. The related molecular mechanisms were assessed by western blotting. We also used the MTT assay and flow cytometry to investigate if silencing of mutant p53 by knockdown with small interfering (si)RNA would change the sensitivity to cisplatin treatment.
Results: Using 5637 and T24 human bladder cancer cell lines characterized by mutations in p53, we found that silencing of the mutant p53 by RNA interference induced evident inhibition of cell proliferation and viability, which was related to the induction of G2 phase cell cycle arrest and apoptosis. Moreover, our study also showed that the p53-targeting siRNA cooperated with cisplatin in the inhibition of bladder cancer cells.
Conclusions: These findings suggest that RNA interference targeting mutant p53 may be a promising therapeutic strategy for the treatment of bladder cancer.
Figures
Figure 1
A p53-targeting small interfering (si)RNA (siP53) reduced p53 expression in T24 and 5637 human bladder cancer cells. p53 mRNA expression in (A) 5637 and (C) T24 cells transfected by siP53 or siCon were assessed by real-time quantitative PCR. The results were normalized to GAPDH and are presented as the mean ± SD of three independent experiments. * P < 0.05 versus mock and small interfering control (siCon) groups. The p53 protein levels in (B) 5637 and (D) T24 cells were assessed by western blotting analysis. β-actin levels were also assessed and served as a loading control. A representative blot is shown from three independent experiments with identical results.
Figure 2
The p53-targeting small interfering (si)RNA (siP53) inhibited the growth of cells. (A) 5637 and (B) T24 cells were transfected with 50 nmol/l siP53, 50 nmol/l siCon or mock RNA. Cell images were taken at 48 hours after transfection at 100× magnification. The siP53-transfected cells were less dense and had more dead cells in the culture than the cells in the siCon and mock groups.
Figure 3
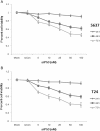
The small interfering (si)P53 inhibited the viability of cells in a dose-dependent and time-dependent manner, as assessed by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For both (A) 5637 and (B) T24 cells, reduced cell viability was seen after siP53 treatment (5 to 100 nmol/l) at 24, 48, and 72 hours. Data are presented as means ± SD (n = 8).
Figure 4
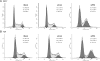
Treatment with 50 nmol/l small interfering (si)P53 induced G2-phase cell cycle arrest in human bladder cancer cells detected by flow cytometry. (A) 5637 and (B) T24 cells. The sub-G0/G1 cells were not included in the calculations. A representative image is shown from three independent experiments with identical results, and these data are the average results of three independent experiments.
Figure 5
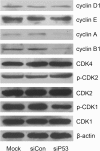
The effects of small interfering (si)P53 on cell cycle-related proteins in 5637 cells. Cell lysates were analyzed by immunoblotting. A representative blot is shown from three independent experiments with identical results.
Figure 6
Small interfering (si)P53-induced apoptosis in 5637 human bladder cancer cells. (A) siP53 treatment resulted in apoptosis in 5637 cells detected by flow cytometry using a double-staining method with fluorescein thiocyanate-conjugated annexin V and propidium iodide. (B) siP53 treatment activated caspase-3, caspase-9, and poly(ADP-ribose) polymerase (PARP) in 5637 cells. Representative images and blots from three independent experiments with identical results are shown, and these data are the average results of three independent experiments.
Figure 7
Silencing of mutant p53 cooperates with cisplatin in inhibiting the viability and growth of 5637 cells assessed by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. These data are presented as means ± SD (n = 8). * P < 0.05 versus single treatment with the conrol (siCon) or mock DNA. ** P < 0.05 versus single treatment with siP53 + cisplatin.
Figure 8
Silencing of mutant p53 cooperates with cisplatin in inducing apoptosis in 5637 cells assessed by flow cytometry. The percentage of cells in G1, S, or G2 phase equals the number of cells in each phase divided by the number in all three phases, and the percentage of sub-G0/G1 cells equals the number of sub-G0/G1 cells divided by the number of all cells counted. A representative image is shown from three independent experiments with identical results, and these data are the average results of three independent experiments.
Similar articles
- Tumour suppressive effects of WEE1 gene silencing in breast cancer cells.
Ghiasi N, Habibagahi M, Rosli R, Ghaderi A, Yusoff K, Hosseini A, Abdullah S, Jaberipour M. Ghiasi N, et al. Asian Pac J Cancer Prev. 2014 Jan;14(11):6605-11. doi: 10.7314/apjcp.2013.14.11.6605. Asian Pac J Cancer Prev. 2014. PMID: 24377575 - Chaetoglobosin K induces apoptosis and G2 cell cycle arrest through p53-dependent pathway in cisplatin-resistant ovarian cancer cells.
Li B, Gao Y, Rankin GO, Rojanasakul Y, Cutler SJ, Tu Y, Chen YC. Li B, et al. Cancer Lett. 2015 Jan 28;356(2 Pt B):418-33. doi: 10.1016/j.canlet.2014.09.023. Epub 2014 Oct 7. Cancer Lett. 2015. PMID: 25304379 Free PMC article. - Radiation induces p53-dependent cell apoptosis in bladder cancer cells with wild-type- p53 but not in p53-mutated bladder cancer cells.
Hinata N, Shirakawa T, Zhang Z, Matsumoto A, Fujisawa M, Okada H, Kamidono S, Gotoh A. Hinata N, et al. Urol Res. 2003 Dec;31(6):387-96. doi: 10.1007/s00240-003-0355-9. Epub 2003 Sep 4. Urol Res. 2003. PMID: 12955365 - The p53 tumor suppressor gene and nuclear protein: basic science review and relevance in the management of bladder cancer.
Smith ND, Rubenstein JN, Eggener SE, Kozlowski JM. Smith ND, et al. J Urol. 2003 Apr;169(4):1219-28. doi: 10.1097/01.ju.0000056085.58221.80. J Urol. 2003. PMID: 12629332 Review. - Tumor suppressor p53 and its gain-of-function mutants in cancer.
Liu J, Zhang C, Feng Z. Liu J, et al. Acta Biochim Biophys Sin (Shanghai). 2014 Mar;46(3):170-9. doi: 10.1093/abbs/gmt144. Epub 2013 Dec 29. Acta Biochim Biophys Sin (Shanghai). 2014. PMID: 24374774 Free PMC article. Review.
Cited by
- Molecular targets in urothelial cancer: detection, treatment, and animal models of bladder cancer.
Smolensky D, Rathore K, Cekanova M. Smolensky D, et al. Drug Des Devel Ther. 2016 Oct 5;10:3305-3322. doi: 10.2147/DDDT.S112113. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27784990 Free PMC article. Review. - Apoptotic effect of the selective PPARβ/δ agonist GW501516 in invasive bladder cancer cells.
Péchery A, Fauconnet S, Bittard H, Lascombe I. Péchery A, et al. Tumour Biol. 2016 Nov;37(11):14789-14802. doi: 10.1007/s13277-016-5305-6. Epub 2016 Sep 16. Tumour Biol. 2016. PMID: 27638828 - Mechanisms of radiation toxicity in transformed and non-transformed cells.
Panganiban RA, Snow AL, Day RM. Panganiban RA, et al. Int J Mol Sci. 2013 Jul 31;14(8):15931-58. doi: 10.3390/ijms140815931. Int J Mol Sci. 2013. PMID: 23912235 Free PMC article. Review. - P53 codon 72 Arg/Pro polymorphism and glioma risk: an updated meta-analysis.
He F, Xia Y, Liu H, Li J, Wang C. He F, et al. Tumour Biol. 2013 Oct;34(5):3121-30. doi: 10.1007/s13277-013-0880-2. Epub 2013 Jul 17. Tumour Biol. 2013. PMID: 23860773 - Molecular crosstalk between cancer and neurodegenerative diseases.
Seo J, Park M. Seo J, et al. Cell Mol Life Sci. 2020 Jul;77(14):2659-2680. doi: 10.1007/s00018-019-03428-3. Epub 2019 Dec 28. Cell Mol Life Sci. 2020. PMID: 31884567 Free PMC article. Review.
References
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP Jr, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. - DOI - PubMed
- Splinter TA, Scher HI, Denis L, Bukowski R, Simon S, Klimberg I, Soloway M, Vogelzang NJ, van Tinteren H, Herr H. European Organization for Research on Treatment of Cancer-Genitourinary Group. The prognostic value of the pathological response to combination chemotherapy before cystectomy in patients with invasive bladder cancer. J Urol. 1992;147:606–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous