The dynamic process of β(2)-adrenergic receptor activation - PubMed (original) (raw)
. 2013 Jan 31;152(3):532-42.
doi: 10.1016/j.cell.2013.01.008.
Yaozhong Zou, Ron O Dror, Thomas J Mildorf, Daniel H Arlow, Aashish Manglik, Albert C Pan, Corey W Liu, Juan José Fung, Michael P Bokoch, Foon Sun Thian, Tong Sun Kobilka, David E Shaw, Luciano Mueller, R Scott Prosser, Brian K Kobilka
Affiliations
- PMID: 23374348
- PMCID: PMC3586676
- DOI: 10.1016/j.cell.2013.01.008
The dynamic process of β(2)-adrenergic receptor activation
Rie Nygaard et al. Cell. 2013.
Abstract
G-protein-coupled receptors (GPCRs) can modulate diverse signaling pathways, often in a ligand-specific manner. The full range of functionally relevant GPCR conformations is poorly understood. Here, we use NMR spectroscopy to characterize the conformational dynamics of the transmembrane core of the β(2)-adrenergic receptor (β(2)AR), a prototypical GPCR. We labeled β(2)AR with (13)CH(3)ε-methionine and obtained HSQC spectra of unliganded receptor as well as receptor bound to an inverse agonist, an agonist, and a G-protein-mimetic nanobody. These studies provide evidence for conformational states not observed in crystal structures, as well as substantial conformational heterogeneity in agonist- and inverse-agonist-bound preparations. They also show that for β(2)AR, unlike rhodopsin, an agonist alone does not stabilize a fully active conformation, suggesting that the conformational link between the agonist-binding pocket and the G-protein-coupling surface is not rigid. The observed heterogeneity may be important for β(2)AR's ability to engage multiple signaling and regulatory proteins.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
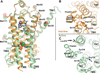
Figure 1. The β2AR is a versatile signaling protein
A) The β2AR interacts with several signaling and regulatory proteins in an agonist-dependent manner. The β2AR can activate the heterotrimeric G proteins Gs and Gi. G-protein coupled receptor kinases (GRKs) phosphorylate the agonist-bound receptor, which can subsequently bind to arrestin and be either internalized or signal through the MAP kinase and other pathways. B) The β2AR exhibits basal agonist-independent activation of Gs. Drugs that can suppress basal activity are called inverse agonists (for example carazolol). Neutral antagonists (for example alprenolol) can block binding of other ligands, but don’t impose any biological response. Agonists can be divided into two categories: full agonists (for example BI-167107) and partial agonists. Full agonists produce the full biological response, whereas partial agonists can only produce a partial biological response even at saturating concentrations. These properties are independent of ligand affinity. C) Schematic free energy landscapes illustrating the energy of the receptor along the activation pathway. The top, middle, and bottom panels show the energy landscape with no ligand bound, with agonist bound, and with both agonist and nanobody 80 (Nb80) bound, respectively. The middle and bottom panels also show the unliganded landscape as a dashed line for comparison. D) The NMR experiments show that agonist binding to the β2AR does not fully stabilize the active conformation suggesting a relatively weak conformational link between the agonist binding pocket and the G protein coupling surface. Results from crystal structures of Metarhodopsin II and double electron-electron resonance spectroscopy suggest that covalently bound trans-retinal can stabilize the active state of the G protein coupling surface.
Figure 2. Positions of methionines in active and inactive β2AR structures
A) Methionine residues shown as sticks in the active and inactive β2AR crystal structures. Solid spheres represent the methionine methyl carbons left in β2AR-Δ5, whereas dotted spheres represent methyls of methionines mutated to other residues in β2AR-Δ5. The functional properties of β2AR-Δ5 are similar to those of wild-type β2AR (See also Fig. S1). B) Structure of β2AR in the crystallographic inactive conformation (2RH1) seen from the intracellular side. Leu2726.34 and surrounding hydrophobic residues are shown as sticks. C) Structure of β2AR in the crystallographic active conformation (3P0G) seen from the intracellular side.
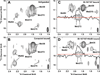
Figure 3. Ligand-specific effects on the HSQC spectrum of 13C-Met-β2AR-Δ5
Carbon-HSQC of spectra of β2AR-Δ5 were obtained under the following conditions: A) unliganded, B) bound to the inverse agonist carazolol, C) bound to the agonist BI-167107, and D) bound to BI-167107 and the G protein mimetic Nb80. In contrast to the complex spectra of unmodified β2AR containing nine methionines (See Fig. S2), resonances for individual methionines are clearly distinguishable in the spectra for β2AR-Δ5. Assignments of 13C-Met resonances in β2AR-Δ5 were made by obtaining spectra of β2AR-Δ5+M36L, β2AR-Δ5+M82V and β2AR-Δ5+M215I (See Fig. S3). Spectra A–C were recorded at room temperature on a 900 MHz Bruker spectrometer. Spectrum D was recorded at room temperature on an 800 MHz Varian spectrometer. See Table S2 for details about acquisition of NMR spectra. In all spectra, a peak at 1.4 ppm [1H] and 19.2 ppm [13C] is observed, we expect this peak represents non-methionine methyl groups in the receptor that are observed because of the natural abundance 13C in the sample.
Figure 4. Ligand-specific effects on the HSQC spectrum of 13C-Met-β2AR-Δ5-L272M
Carbon-HSQC spectra of β2AR-Δ5-L272M were obtained under the following conditions: A) unliganded, B) bound to the inverse agonist carazolol, C) bound to the agonist BI-167107, and D) bound to BI-167107 and the G protein mimetic Nb80. Spectra were recorded at room temperature on an 800 MHz Varian spectrometer (see Table S2 for details about acquisition of NMR spectra). For the spectra with BI-167107 and BI-167107 plus Nb80 bound a 1D slice illustrates the splitting of the Met2726.34. The red line represents the carbon chemical shift where this 1D slice was taken.
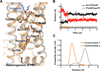
Figure 5. Alternative inactive state observed in unliganded and inverse-agonist-bound MD simulations may explain dual Met822.53 peaks
A) The transition from the crystallographic inactive conformation (orange, 2RH1) to the alternative inactive conformation (gray, snapshot from MD simulation) involves a rotation of the intracellular part of TM7. Pro3237.50 moves ~4 Å towards TM2 into the space between Asp792.50 and Asn511.50, displacing a conserved water molecule linking these two residues (Pardo et al., 2007), and Tyr3267.53 adopts the trans χ1 rotamer, pointing its phenol hydroxyl down towards the ionic lock. Ser3197.46 shifts ~2 Å towards Trp2866.48 and forms a hydrogen bond, and its displacement opens a small hydrophobic cavity at the interface of TM1, TM2, and TM7 into which Met822.53’s ε-methyl group docks. B) Distances between the alpha-carbon atoms of Ser3197.46 and Ile471.46 (black) and Pro3237.50 and Leu757.46 (red) over the course of a simulation of β2AR which transitions from the crystallographic inactive conformation to the alternative inactive conformation after 1.93 µs. The bar at the bottom of the plot illustrates when we see the crystallographic inactive conformation (orange) and the alternative inactive conformation 2 (gray). C) Distributions of the distance from Cε of Met822.53 to the closest non-hydrogen atom in the aromatic ring of Trp2866.48, plotted for the crystallographic inactive and alternative inactive conformation. See Fig. S4 for additional analysis these two inactive conformations, and Table S3 for additional details about molecular dynamics experiments.
Figure 6. Agonist binding promotes conformational heterogeneity around Met2155.54 and Met2796.41
A) Peak volumes for individual methionines. Automated 2D line-shape fitting was performed on the spectra using NMRpipe (Delaglio et al., 1995). From the line-shape analysis volumes of the different peaks were extracted. The peak volumes were normalized to the peak volume of Met36. After extracting peak volumes we estimated their uncertainties by evaluating the RMS from the volumes of 10 peaks chosen from regions of the spectrum where no signal could be detected in the 2D spectrum. For Met82 the peak volumes in the carazolol and the unliganded state are averages of peak intensities of the two most intense Met82 peaks. Although NB80 would be expected to reduce tumbling of the receptor in solution and thereby weaken signals, we see signals intensify suggesting a stabilization of the receptor and a more uniform distribution of conformations compared to the agonist bound form (see also Fig. S5). B) Region around Met2155.54 and Met2796.41 shown for the active (green, 3P0G) and inactive (orange, 2RH1) structures of β2AR. Met2155.54 and Met2796.41 and aromatic residues in the vicinity of Met2155.54 and Met2796.41 are shown as sticks. Arg1313.50 and Leu2726.34 are also shown as sticks. C) Simulations of β2AR starting in the active conformation with Nb80 removed spontaneously transition back to the inactive conformation. Top two plots illustrate a transition from the active conformation to the inactive conformation during an MD simulation (adapted from Fig. 2 of Dror et al. (2011a)). The transition starts with the re-arrangement of TM7 into its inactive conformation, as illustrated by the plot of TM7’s RMSD to the inactive crystal structure; subsequently TM6 moves inward towards TM3, as illustrated by the plot of distances between TM3 (Cα of Arg1313.50) and TM6 (Cα of Leu2726.34). We call the state between the re-arrangement of TM7 and the inward movement of TM6 the intermediate state. The bar in the bottom of the plot illustrates what part of the simulation is considered the active (green), intermediate (blue) and inactive (orange) state. The bottom four plots illustrate the distance distributions during the simulation between two NMR probes (Met2155.54 or Met2796.41) and two nearby aromatic residues (Tyr2195.58 or Phe2826.44). The distance distribution of the active conformation is based on simulations of β2AR with BI-167107 and Nb80 bound; the inactive state is based on simulations of the carazolol bound receptor, and the intermediate state is based on simulations with only BI-167107 bound, as illustrated in the two top plots. See Table S3 for additional details about molecular dynamics experiments.
Figure 7. Conformational diversity in inactive, active and intermediate states
Snapshots from MD simulations are shown every 18 ns. Met2155.54, Met2796.41, Tyr2195.58 and Phe2826.44 are shown as sticks. The top panel shows snapshots from a crystallographic- inactive-state simulation with carazolol bound. The middle panel shows snapshots from an active-state simulation with BI-167107 and Nb80 bound. The bottom panel shows frames from the intermediate state (blue), with only BI-167107 bound. In the bottom panel we also show the backbone of crystallographic inactive (orange) and active (green) states.
Comment in
- Conformational ensembles in GPCR activation.
Vardy E, Roth BL. Vardy E, et al. Cell. 2013 Jan 31;152(3):385-6. doi: 10.1016/j.cell.2013.01.025. Cell. 2013. PMID: 23374334
Similar articles
- Identifying ligand binding conformations of the β2-adrenergic receptor by using its agonists as computational probes.
Isin B, Estiu G, Wiest O, Oltvai ZN. Isin B, et al. PLoS One. 2012;7(12):e50186. doi: 10.1371/journal.pone.0050186. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300522 Free PMC article. - Identifying conformational changes of the beta(2) adrenoceptor that enable accurate prediction of ligand/receptor interactions and screening for GPCR modulators.
Reynolds KA, Katritch V, Abagyan R. Reynolds KA, et al. J Comput Aided Mol Des. 2009 May;23(5):273-88. doi: 10.1007/s10822-008-9257-9. Epub 2009 Jan 16. J Comput Aided Mol Des. 2009. PMID: 19148767 Free PMC article. - Insight into partial agonism by observing multiple equilibria for ligand-bound and Gs-mimetic nanobody-bound β1-adrenergic receptor.
Solt AS, Bostock MJ, Shrestha B, Kumar P, Warne T, Tate CG, Nietlispach D. Solt AS, et al. Nat Commun. 2017 Nov 27;8(1):1795. doi: 10.1038/s41467-017-02008-y. Nat Commun. 2017. PMID: 29176642 Free PMC article. - Structural features of β2 adrenergic receptor: crystal structures and beyond.
Bang I, Choi HJ. Bang I, et al. Mol Cells. 2015;38(2):105-11. doi: 10.14348/molcells.2015.2301. Epub 2014 Dec 24. Mol Cells. 2015. PMID: 25537861 Free PMC article. Review. - Structural insights into agonist-induced activation of G-protein-coupled receptors.
Deupi X, Standfuss J. Deupi X, et al. Curr Opin Struct Biol. 2011 Aug;21(4):541-51. doi: 10.1016/j.sbi.2011.06.002. Epub 2011 Jun 30. Curr Opin Struct Biol. 2011. PMID: 21723721 Review.
Cited by
- Advances in receptor conformation research: the quest for functionally selective conformations focusing on the β2-adrenoceptor.
Woo AY, Song Y, Zhu W, Xiao RP. Woo AY, et al. Br J Pharmacol. 2015 Dec;172(23):5477-88. doi: 10.1111/bph.13049. Epub 2015 Feb 27. Br J Pharmacol. 2015. PMID: 25537131 Free PMC article. Review. - Differential Behavior of Conformational Dynamics in Active and Inactive States of Cannabinoid Receptor 1.
Isu UH, Polasa A, Moradi M. Isu UH, et al. J Phys Chem B. 2024 Sep 5;128(35):8437-8447. doi: 10.1021/acs.jpcb.4c02828. Epub 2024 Aug 22. J Phys Chem B. 2024. PMID: 39169808 Free PMC article. - Sensing conformational changes in metabotropic glutamate receptors.
Rives ML, Javitch JA. Rives ML, et al. Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):5742-3. doi: 10.1073/pnas.1303736110. Epub 2013 Mar 29. Proc Natl Acad Sci U S A. 2013. PMID: 23542381 Free PMC article. No abstract available. - Challenges in structural approaches to cell modeling.
Im W, Liang J, Olson A, Zhou HX, Vajda S, Vakser IA. Im W, et al. J Mol Biol. 2016 Jul 31;428(15):2943-64. doi: 10.1016/j.jmb.2016.05.024. Epub 2016 May 30. J Mol Biol. 2016. PMID: 27255863 Free PMC article. Review. - Revealing the favorable dissociation pathway of type II kinase inhibitors via enhanced sampling simulations and two-end-state calculations.
Sun H, Tian S, Zhou S, Li Y, Li D, Xu L, Shen M, Pan P, Hou T. Sun H, et al. Sci Rep. 2015 Feb 13;5:8457. doi: 10.1038/srep08457. Sci Rep. 2015. PMID: 25678308 Free PMC article.
References
- Beatty EJ, Cox MC, Frenkiel TA, Tam BM, Mason AB, MacGillivray RT, Sadler PJ, Woodworth RC. Interlobe communication in 13C-methionine-labeled human transferrin. Biochemistry. 1996;35:7635–7642. - PubMed
- Bose-Basu B, DeRose EF, Kirby TW, Mueller GA, Beard WA, Wilson SH, London RE. Dynamic characterization of a DNA repair enzyme: NMR studies of [methyl-13C]methionine-labeled DNA polymerase beta. Biochemistry. 2004;43:8911–8922. - PubMed
- Choe HW, Kim YJ, Park JH, Morizumi T, Pai EF, Krauss N, Hofmann KP, Scheerer P, Ernst OP. Crystal structure of metarhodopsin II. Nature. 2011;471:651–655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials