Retinoid signaling alterations in amyotrophic lateral sclerosis - PubMed (original) (raw)
Retinoid signaling alterations in amyotrophic lateral sclerosis
Christi L Kolarcik et al. Am J Neurodegener Dis. 2012.
Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neuromuscular disease for which effective therapeutic interventions and an understanding of underlying disease mechanism are lacking. A variety of biochemical pathways are believed to contribute to the pathophysiology of ALS that are common to both sporadic and familial forms of the disease. Evidence from both human and animal studies indicates that expression of retinoid signaling genes is altered in ALS and may contribute to motor neuron loss. Our goals were to examine the expression and distribution of proteins of the retinoid signaling pathway in spinal cord samples from patients with sporadic and familial ALS and to evaluate the role of these proteins in motor neuron cell survival. In sporadic ALS, the cytoplasmic binding protein that facilitates nuclear translocation of retinoic acid, cellular retinoic acid binding protein-II (CRABP-II), was localized to the nucleus and retinoic acid receptor β (RARβ) was significantly increased in motor neuron nuclei when compared to either familial ALS patients or non-neurologic disease controls. Motor neurons with increased nuclear RARβ were negative for markers of apoptosis. Pre-treatment of primary motor neuron-enriched cultures with a pan-RAR or RARβ-specific agonist decreased motor neuron cell death associated with oxidative injury/stress while a RARβ-specific antagonist enhanced cell death. Our data suggest retinoid signaling is altered in ALS and increased nuclear RARβ occurs in motor neurons of sporadic ALS patients. Activation of RARβ protects motor neurons from oxidative-induced cell death.
Keywords: Retinoid signaling; amyotrophic lateral sclerosis; motor neuron; motor neurons; nuclear receptor; oxidative stress-induced cell death; retinoic acid receptors.
Figures
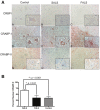
Figure 1
Immunohistochemical analysis of cytoplasmic binding proteins of the retinoic acid signaling pathway in human spinal cord tissue. A: Lumbar spinal cord sections were immunostained for CRBPI (A-C), CRABP-I (D-F) and CRABP-II (G-I) in red and counterstained with hematoxylin. Insets represent a high power magnification of each panel. Original magnifications: 200X (A to I); 400X (insets in A to I). B: The localization of CRABP-II was assessed for morphologically distinct motor neurons from each patient group. The percent of motor neurons with nuclear CRABP-II was significantly greater in patients with SALS (white bars) as compared to FALS (black bars; p < 0.01) or controls (stippled bars; _p_ < 0.001). There was no significant difference between FALS patients and controls (_p_ > 0.05).
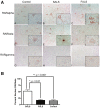
Figure 2
Immunohistochemical analysis of retinoic acid receptor (RAR) proteins in human spinal cord tissue. A: Lumbar spinal cord sections were immunostained for RARα (A-C), RARβ (D-F) and RARγ (G-I) in red and counterstained with hematoxylin. Insets represent a high power magnification of each panel. Original magnifications: 200X (A to I); 400X (insets in A to I). B: Nuclear RARβ immunoreactivity was determined by counting morphologically distinct motor neurons from each patient group. The percent of motor neurons with increased nuclear RARβ was significantly greater in patients with SALS (white bars) when compared to FALS (black bars; p < 0.01) or controls (stippled bars; _p_ < 0.001). There was no significant difference between FALS patients and controls (_p_ > 0.05).
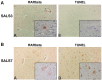
Figure 3
Immunohistochemical analysis of RARβ and TUNEL in human spinal cord. Serial lumbar spinal cord sections from SALS cases were immunostained for RARβ and TUNEL and representative cases are shown. Identical motor neurons were imaged as determined by anatomical hallmarks and location within the tissue. A: Motor neurons with intense nuclear RARβ immunostaining (A) lacked TUNEL staining (B). B: Motor neurons within the spinal cord without nuclear RARβ immunostaining (A) were TUNEL positive (B). Insets represent a high power magnification of each panel. Original magnifications: 200X; 400X (insets).
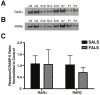
Figure 4
Protein-protein interactions in nuclear-enriched fractions from human lumbar spinal cord. Protein extracts from ALS and controls were prepared as described in the Materials and Methods. Protein (500 μg) from each extract was immunoprecipitated with CRABP-II and resulting blots were probed with antibodies specific to RARα (A) or RARβ (B). A: Immunoblots of nuclear-enriched fractions from lumbar spinal cord co-immunoprecipitated with CRABP-II and probed for RARα. B: Immunoblots of nuclear-enriched fractions from lumbar spinal cord coimmunoprecipitated with CRABP-II and probed for RARβ. C: Graph representing the results from densitometry quantification. RAR Receptor levels were normalized to that of CRABP-II for each sample. Black bars represent control cases (n = 2) and checkered bars are ALS cases (n = 6). Statistical analyses were performed using an unpaired t-test with a 95% confidence interval. The p values for RARα and RARβ were 0.9042 and 0.8881, respectively. Letters and numbers correspond to patients in Table 1.
Figure 5
In vitro expression of the RARs. Expression of the three RAR isotypes was evaluated in the motor neuron-enriched cell culture system. Polymerase chain reaction (PCR) analysis of RNA isolated from primary motor neuron cultures after 24 hours in culture. RARα and RARβ but not RARγ were expressed. “MN” denotes RNA from primary motor neurons; “+” denotes RNA isolated from JM2 cells used as a positive control. Cyclophilin was used as a PCR positive control for each cell type.
Figure 6
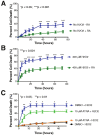
The presence of RA modulates cell death. The percentage of motor neuron cell death was evaluated using live cell imaging and propidium iodide staining over time. A: The absence of RA in the B27 media supplement (blue curve) resulted in significantly increased cell death in the absence of any insult. B: When treated with H2O2 to model oxidative stress/injury, the absence of RA in the B27 media supplement (blue curve) resulted in significantly increased percentages of cell death beginning at the 6.0 hour time point. C: Motor neuron-enriched cultures were pre-treated with ATRA for 1.0 hour prior to addition of H2O2 to induce cell death. In the presence of H2O2, ATRA significantly decreased the percentage of cell death as compared to the control treatment (DMSO + H2O2). Control treatments without H2O2 (ATRA or DMSO alone) were included for comparison (orange and black curves). Error bars represent the mean percentage of cell death ± standard error of the mean from at least two separate experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 7
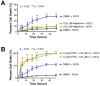
RARβ activity regulates H2O2 induced cell death. Motor neuron cultures were pre-treated for 1.0 hour with agents that specifically target RARβ prior to the addition of toxin. A: In the presence of adapalene, a RARβ agonist, the percentage of cell death induced by H2O2 was significantly reduced when compared to the control treatment of DMSO plus H2O2 (compare blue and green curves). Controls of DMSO alone or adapalene alone measured baseline level of cell death over time (black and orange curves). B: Pre-treatment of cells with a combination of ATRA and LE-135, a selective RARβ antagonist, eliminated the protective effects of ATRA and exacerbated the toxicity in the presence or absence of H2O2 (green and orange curves). Control treatments of DMSO alone (black curve) or DMSO plus H2O2 (blue curve) are shown. Error bars represent the mean percentage of cell death ± standard error of the mean from at least two separate experiments. *p < 0.05; ***p < 0.001.
Figure 8
Schematic representation of retinoid signaling in SALS. Increased nuclear localization of RARβ occurs in SALS. Nuclear CRABP-II and cytoplasmic RARα localization were also observed. Signaling through RARβ in vitro was neuroprotective in the presence of oxidative stress. Processes shown to be mediated by the RARs are provided as potential downstream targets mediating this protection. Pharmacologic agents that stimulate RARβ may be promising therapeutic targets that could delay or prevent motor neuron cell death in ALS.
Similar articles
- Retinoid receptors in chronic degeneration of the spinal cord: observations in a rat model of amyotrophic lateral sclerosis.
Jokic N, Ling YY, Ward RE, Michael-Titus AT, Priestley JV, Malaspina A. Jokic N, et al. J Neurochem. 2007 Dec;103(5):1821-33. doi: 10.1111/j.1471-4159.2007.04893.x. Epub 2007 Sep 13. J Neurochem. 2007. PMID: 17956549 - The role of autophagy: what can be learned from the genetic forms of amyotrophic lateral sclerosis.
Pasquali L, Ruffoli R, Fulceri F, Pietracupa S, Siciliano G, Paparelli A, Fornai F. Pasquali L, et al. CNS Neurol Disord Drug Targets. 2010 Jul;9(3):268-78. doi: 10.2174/187152710791292594. CNS Neurol Disord Drug Targets. 2010. PMID: 20406184 Review. - Macrophage-mediated inflammation and glial response in the skeletal muscle of a rat model of familial amyotrophic lateral sclerosis (ALS).
Van Dyke JM, Smit-Oistad IM, Macrander C, Krakora D, Meyer MG, Suzuki M. Van Dyke JM, et al. Exp Neurol. 2016 Mar;277:275-282. doi: 10.1016/j.expneurol.2016.01.008. Epub 2016 Jan 13. Exp Neurol. 2016. PMID: 26775178 Free PMC article. - Persistent cleavage and nuclear translocation of apoptosis-inducing factor in motor neurons in the spinal cord of sporadic amyotrophic lateral sclerosis patients.
Shibata N, Kakita A, Takahashi H, Ihara Y, Nobukuni K, Fujimura H, Sakoda S, Sasaki S, Yamamoto T, Kobayashi M. Shibata N, et al. Acta Neuropathol. 2009 Dec;118(6):755-62. doi: 10.1007/s00401-009-0580-6. Acta Neuropathol. 2009. PMID: 19669652 - A review of the functional role and of the expression profile of retinoid signaling and of nuclear receptors in human spinal cord.
Malaspina A, Turkheimer F. Malaspina A, et al. Brain Res Bull. 2007 Mar 15;71(5):437-46. doi: 10.1016/j.brainresbull.2006.10.032. Epub 2006 Dec 1. Brain Res Bull. 2007. PMID: 17259011 Review.
Cited by
- TDP-43 gains function due to perturbed autoregulation in a Tardbp knock-in mouse model of ALS-FTD.
White MA, Kim E, Duffy A, Adalbert R, Phillips BU, Peters OM, Stephenson J, Yang S, Massenzio F, Lin Z, Andrews S, Segonds-Pichon A, Metterville J, Saksida LM, Mead R, Ribchester RR, Barhomi Y, Serre T, Coleman MP, Fallon JR, Bussey TJ, Brown RH Jr, Sreedharan J. White MA, et al. Nat Neurosci. 2018 Apr;21(4):552-563. doi: 10.1038/s41593-018-0113-5. Epub 2018 Mar 19. Nat Neurosci. 2018. PMID: 29556029 Free PMC article. - Palliative Care Issues in Amyotrophic Lateral Sclerosis: An Evidenced-Based Review.
Karam CY, Paganoni S, Joyce N, Carter GT, Bedlack R. Karam CY, et al. Am J Hosp Palliat Care. 2016 Feb;33(1):84-92. doi: 10.1177/1049909114548719. Epub 2014 Sep 8. Am J Hosp Palliat Care. 2016. PMID: 25202033 Free PMC article. Review. - Nuclear Receptors as Therapeutic Targets for Neurodegenerative Diseases: Lost in Translation.
Moutinho M, Codocedo JF, Puntambekar SS, Landreth GE. Moutinho M, et al. Annu Rev Pharmacol Toxicol. 2019 Jan 6;59:237-261. doi: 10.1146/annurev-pharmtox-010818-021807. Epub 2018 Sep 12. Annu Rev Pharmacol Toxicol. 2019. PMID: 30208281 Free PMC article. Review. - Association of Serum Retinol-Binding Protein 4 Concentration With Risk for and Prognosis of Amyotrophic Lateral Sclerosis.
Rosenbohm A, Nagel G, Peter RS, Brehme T, Koenig W, Dupuis L, Rothenbacher D, Ludolph AC; ALS Registry Study Group. Rosenbohm A, et al. JAMA Neurol. 2018 May 1;75(5):600-607. doi: 10.1001/jamaneurol.2017.5129. JAMA Neurol. 2018. PMID: 29482216 Free PMC article. - Neuroprotective effects of ellorarxine in neuronal models of degeneration.
Kouchmeshky A, Whiting A, McCaffery P. Kouchmeshky A, et al. Front Neurosci. 2024 Sep 10;18:1422294. doi: 10.3389/fnins.2024.1422294. eCollection 2024. Front Neurosci. 2024. PMID: 39376539 Free PMC article.
References
- Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol. 2011;7:603–615. - PubMed
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. - PubMed
- Mey J, McCaffery P. Retinoic acid signaling in the nervous system of adult vertebrates. Neuroscientist. 2004;10:409–421. - PubMed
- Wong LF, Yip PK, Battaglia A, Grist J, Corcoran J, Maden M, Azzouz M, Kingsman SM, Kingsman AJ, Mazarakis ND, McMahon SB. Retinoic acid receptor beta2 promotes functional regeneration of sensory axons in the spinal cord. Nat Neurosci. 2006;9:243–250. - PubMed
Grants and funding
- R01 NS061867/NS/NINDS NIH HHS/United States
- R56 NS061867/NS/NINDS NIH HHS/United States
- T32 EB001026/EB/NIBIB NIH HHS/United States
- TL1 RR024155/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous