Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving - PubMed (original) (raw)
Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving
Florence R Theberge et al. Biol Psychiatry. 2013.
Abstract
Background: Recent evidence implicates toll-like receptor 4 (TLR4) in opioid analgesia, tolerance, conditioned place preference, and self-administration. Here, we determined the effect of the TLR4 antagonist (+)-naltrexone (a μ-opioid receptor inactive isomer) on the time-dependent increases in cue-induced heroin seeking after withdrawal (incubation of heroin craving).
Methods: In an initial experiment, we trained rats for 9 hours per day to self-administer heroin (.1 mg/kg/infusion) for 9 days; lever presses were paired with a 5-second tone-light cue. We then assessed cue-induced heroin seeking in 30-minute extinction sessions on withdrawal day 1; immediately after testing, we surgically implanted rats with Alzet minipumps delivering (+)-naltrexone (0, 7.5, 15, 30 mg/kg/day, subcutaneous) for 14 days. We then tested the rats for incubated cue-induced heroin seeking in 3-hour extinction tests on withdrawal day 13.
Results: We found that chronic delivery of (+)-naltrexone via minipumps during the withdrawal phase decreased incubated cue-induced heroin seeking. In follow-up experiments, we found that acute injections of (+)-naltrexone immediately before withdrawal day 13 extinction tests had no effect on incubated cue-induced heroin seeking. Furthermore, chronic delivery of (+)-naltrexone (15 or 30 mg/kg/day) or acute systemic injections (15 or 30 mg/kg) had no effect on ongoing extended access heroin self-administration. Finally, in rats trained to self-administer methamphetamine (.1 mg/kg/infusion, 9 hours/day, 9 days), chronic delivery of (+)-naltrexone (30 mg/kg/day) during the withdrawal phase had no effect on incubated cue-induced methamphetamine seeking.
Conclusions: The present results suggest a critical role of TLR4 in the development of incubation of heroin, but not methamphetamine, craving.
Published by Elsevier Inc.
Figures
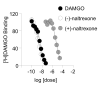
Figure 1. Dose-response curves for inhibition of [3H]DAMGO binding for isomers of naltrexone: (−)-naltrexone and (+)-naltrexone
Membranes from CHO cells expressing human mu opioid receptors were prepared as described in Methods. Ten concentrations of each test drug were incubated in the presence of 3 nM [3H]DAMGO to generate curves. Data are expressed as mean±SD for 3 separate runs performed in triplicates.
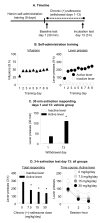
Figure 2. Chronic delivery of (+)-naltrexone during the withdrawal phase decreased incubated cue-induced heroin seeking
(A) Timeline of the experiment. (B)
Heroin self-administration training
. Data are mean±SEM number of heroin infusions (0.1 mg/kg/infusion), and active and inactive lever-presses during the nine 9-h daily self-administration sessions (total n=57). During training, active lever presses were reinforced on an FR1 20-sec timeout reinforcement schedule and heroin infusions were paired with a 5-sec tone-light cue. (C) Extinction test withdrawal day 1 and 13 (vehicle group): Data are mean±SEM of responses on the previously active lever and on the inactive lever in the vehicle-treated rats (n=28) during the 30 min extinction test on withdrawal day 1 and the first 30 min of the 3 h extinction test on withdrawal day 13. * Different from withdrawal day 1, p<0.05. (D)
Extinction test withdrawal day 13:
Data are mean±SEM of responses on the active and inactive levers during the 3-h extinction test. During testing, lever-presses led to contingent presentations of the tone-light cue previously paired with heroin infusions during training, but not heroin. The rats were tested on withdrawal day 13 with Alzet osmotic minimpumps that were implanted s.c. on withdrawal day 1 with either vehicle (sterile water, n=28) or (+)-naltrexone: 7.5, 15, or 30 mg/kg/day (n=9-10 per dose). * Different from vehicle, p<0.05.
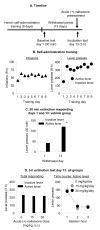
Figure 3. Acute injection of (+)-naltrexone had no effect on incubated cue-induced heroin seeking on withdrawal day 13
(A) Timeline of the experiment. (B)
Heroin self-administration training
. Data are mean±SEM number of heroin infusions (0.1 mg/kg/infusion), and active and inactive lever-presses during the nine 9-h daily self-administration sessions (total n=30). (C) Extinction test withdrawal day 1 and 13 (vehicle group): Data are mean±SEM of responses on the active and inactive levers in the vehicle-treated group (n=10) during the 30 min extinction test on withdrawal day 1 and the first 30 min of the 3 h extinction test on withdrawal day 13. * Different from withdrawal day 1, p<0.05. (D)
Extinction test withdrawal day 13:
Data are mean±SEM responses on the active and inactive levers during the 3-h extinction test. On withdrawal day 13, rats were injected acutely with either vehicle (sterile water, n=10) or (+)-naltrexone (15 or 30 mg/kg, s.c., n=10 per dose) 10-15 min prior to the extinction test.
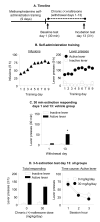
Figure 4. Chronic (minipump) delivery or acute systemic injections of (+)-naltrexone had no effect on ongoing heroin self-administration
A)
Chronic delivery:
Data are total mean±SEM of heroin infusions (0.1 mg/kg/infusion), and active and inactive lever-presses during heroin self-administration training (three 3-h sessions separated by 1 h). Two days prior to training, rats were implanted with s.c. with Alzet osmotic minipumps that delivered either vehicle (sterile water, n=10) or (+)-naltrexone (15 or 30 mg/kg/day, n=8-10 per dose) during the training period. (B)
Acute injections:
Data are mean±SEM of heroin infusions (0.1 mg/kg/infusion) during the first, second, and third daily sessions. Systemic injections (s.c. or i.p.) of (+)-naltrexone (0, 15, and 30 mg/kg; n=10-11) were given 10-15 min prior to the start of the first 3 h daily session.
Figure 5. Chronic delivery of (+)-naltrexone during the withdrawal phase had no effect on incubated cue-induced methamphetamine seeking
(A) Timeline of the experiment. (B)
Methamphetamine self-administration training
. Data are mean±SEM number of methamphetamine infusions (0.1 mg/kg/infusion), and active and inactive lever-presses during the nine 9-h daily self-administration sessions (total n=26). (C) Extinction test withdrawal day 1 and 13 (vehicle group): Data are mean±SEM of responses on the active and inactive levers in the vehicle-treated group (n=13) during the 30 min extinction test on withdrawal day 1 and the first 30 min of the 3-h extinction test on withdrawal day 13. * Different from withdrawal day 1, p<0.05. (D)
Extinction test withdrawal day 13:
Data are mean±SEM responses on the active and inactive levers during the 3-h extinction test. During testing, lever presses led to contingent presentations of the tone-light cue previously paired with methamphetamine infusions during training, but not methamphetamine. The rats were tested on withdrawal day 13 with Alzet osmotic minimpumps that were implanted s.c. on withdrawal day 1 with either vehicle (sterile water, n=13) or (+)-naltrexone, 30 mg/kg/day (n=13).
Similar articles
- Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving.
Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT. Fanous S, et al. J Neurosci. 2012 Aug 22;32(34):11600-9. doi: 10.1523/JNEUROSCI.1914-12.2012. J Neurosci. 2012. PMID: 22915104 Free PMC article. - Effects of training and withdrawal periods on heroin seeking induced by conditioned cue in an animal of model of relapse.
Zhou W, Zhang F, Liu H, Tang S, Lai M, Zhu H, Kalivas PW. Zhou W, et al. Psychopharmacology (Berl). 2009 May;203(4):677-84. doi: 10.1007/s00213-008-1414-2. Epub 2008 Nov 29. Psychopharmacology (Berl). 2009. PMID: 19043694 - Incubation of extinction responding and cue-induced reinstatement, but not context- or drug priming-induced reinstatement, after withdrawal from methamphetamine.
Adhikary S, Caprioli D, Venniro M, Kallenberger P, Shaham Y, Bossert JM. Adhikary S, et al. Addict Biol. 2017 Jul;22(4):977-990. doi: 10.1111/adb.12386. Epub 2016 Mar 14. Addict Biol. 2017. PMID: 26989042 Free PMC article. - Neurobiology of the incubation of drug craving.
Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Pickens CL, et al. Trends Neurosci. 2011 Aug;34(8):411-20. doi: 10.1016/j.tins.2011.06.001. Epub 2011 Jul 20. Trends Neurosci. 2011. PMID: 21764143 Free PMC article. Review. - Voluntary exercise as a treatment for incubated and expanded drug craving leading to relapse to addiction: Animal models.
Carroll ME. Carroll ME. Pharmacol Biochem Behav. 2021 Sep;208:173210. doi: 10.1016/j.pbb.2021.173210. Epub 2021 Jun 8. Pharmacol Biochem Behav. 2021. PMID: 34116079 Review.
Cited by
- Stress- and drug-induced neuroimmune signaling as a therapeutic target for comorbid anxiety and substance use disorders.
Smiley CE, Wood SK. Smiley CE, et al. Pharmacol Ther. 2022 Nov;239:108212. doi: 10.1016/j.pharmthera.2022.108212. Epub 2022 May 14. Pharmacol Ther. 2022. PMID: 35580690 Free PMC article. Review. - Interleukin-1 receptor-associated kinase 4 (IRAK4) in the nucleus accumbens regulates opioid-seeking behavior in male rats.
Wu R, Liu J, Vu J, Huang Y, Dietz DM, Li JX. Wu R, et al. Brain Behav Immun. 2022 Mar;101:37-48. doi: 10.1016/j.bbi.2021.12.014. Epub 2021 Dec 24. Brain Behav Immun. 2022. PMID: 34958862 Free PMC article. - Microglia contribute to methamphetamine reinforcement and reflect persistent transcriptional and morphological adaptations to the drug.
Vilca SJ, Margetts AV, Höglund L, Fleites I, Bystrom LL, Pollock TA, Bourgain-Guglielmetti F, Wahlestedt C, Tuesta LM. Vilca SJ, et al. Brain Behav Immun. 2024 Aug;120:339-351. doi: 10.1016/j.bbi.2024.05.038. Epub 2024 Jun 3. Brain Behav Immun. 2024. PMID: 38838836 Free PMC article. - Neuroimmune mechanisms of psychostimulant and opioid use disorders.
Hofford RS, Russo SJ, Kiraly DD. Hofford RS, et al. Eur J Neurosci. 2019 Aug;50(3):2562-2573. doi: 10.1111/ejn.14143. Epub 2018 Sep 26. Eur J Neurosci. 2019. PMID: 30179286 Free PMC article. Review. - Preventing incubation of drug craving to treat drug relapse: from bench to bedside.
Liu X, Yuan K, Lu T, Lin X, Zheng W, Xue Y, Shi J, Lu L, Han Y. Liu X, et al. Mol Psychiatry. 2023 Apr;28(4):1415-1429. doi: 10.1038/s41380-023-01942-2. Epub 2023 Jan 16. Mol Psychiatry. 2023. PMID: 36646901 Review.
References
- Jaffe JH. Drug addiction and drug abuse. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 8th ed Pergamon Press; New York: 1990. pp. 522–573.
- Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. - PubMed
- O’Brien CP, Ehrman RN, Ternes JW. Classical conditioning in human opioid dependence. In: Goldberg S, Stolerman I, editors. Behavioral analysis of drug dependence. Academic Press; Orlando: 1986. pp. 329–356.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials