Akt and c-Myc induce stem-cell markers in mature primary p53⁻/⁻ astrocytes and render these cells gliomagenic in the brain of immunocompetent mice - PubMed (original) (raw)
Akt and c-Myc induce stem-cell markers in mature primary p53⁻/⁻ astrocytes and render these cells gliomagenic in the brain of immunocompetent mice
Josefine Radke et al. PLoS One. 2013.
Erratum in
- PLoS One. 2013;8(10). doi:10.1371/annotation/562d2594-464f-49f1-90ef-f78cafcd3956
Abstract
Astrocytomas and their most malignant variant glioblastoma multiforme (GBM) represent the vast majority of primary brain tumors. Despite the current progress in neurosurgery, radiation therapy and chemotherapy, most astrocytomas remain fatal disorders. Although brain tumor biology is a matter of intense research, the cell-of-origin and the complete astrocytoma-inducing signaling pathway remain unknown. To further identify the mechanisms leading to gliomagenesis, we transduced primary astrocytes on a p53(-/-) background with c-Myc, constitutively active myr-Akt or both, myr-Akt and c-Myc. Transduced astrocytes showed oncogene-specific alterations of morphology, proliferation and differentiation. Following prolonged periods of cultivation, oncogene-transduced astrocytes expressed several stem-cell markers. Furthermore, astrocytes coexpressing c-Myc and Akt were tumorigenic when implanted into the brain of immunocompetent C57BL/6 mice. Our results reveal that the loss of p53 combined with oncogene overexpression in mature astrocytes simulates pivotal features of glioma pathogenesis, providing a good model for assessing the development of secondary glioblastomas.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
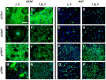
Figure 1. Phenotype of astrocytes expressing constitutively active Akt and/or c-Myc.
The p53Ctrl astrocytes of the 2nd (A) and the 7th (B) passage revealed strong GFAP-immunoreactivity (-IR). In p53AKT astrocytes the GFAP-IR was significantly downregulated as early as the 2nd passage and stayed low through the (E) 8th passage (F). The p53MYC astrocytes showed strong GFAP-IR in the 2nd passage (I) while there was only low GFAP-IR in the 8th passage (J). Astrocytes expressing Akt and c-Myc showed scattered cells with and without GFAP-IR in the 2nd passage (M) and virtually complete loss of GFAP-IR at later passages (8th passage, N). The number of Ki67-IR cells was low in the 2nd passage (C) and slightly increased in the 8th passage (D).The Ki67-IR of p53AKT astrocytes was comparable to p53Ctrl astrocytes at both time points (2nd (G); 8th (H) passage, respectively). Expression of c-Myc immediately induced a strong increase in the Ki67-IR (2nd passage, K) that further increased to the 8th passage (L). The p53MA astrocytes showed a high number of Ki67-IR nuclei (2nd: O; 8th: P) in both passages. (Scale bar: 50 µm)
Figure 2. GFAP and c-Myc gene expression in astrocytes.
A: RPA was performed with 2.5 µg of total RNA of three independent astrocyte cultures for each condition. Constitutively expressed ribosomal Protein L32 RNA was used as a loading control. N: Negative control (tRNA). Autoradiographs were scanned and quantitatively analyzed using the ImageJ (1.42q) program. Strong c-Myc-specific mRNA expression (A, arrow) was observed in p53MA and p53MYC cultures (A, C). The RPA probe for c-myc revealed a weak constitutive band (A, arrowhead) in the RNA from cells that were not transduced for c-Myc (p53Ctrl and p53AKT). GFAP-specific mRNA was detected in p53Ctrl (because of partial degradation of the RNA one sample could not be quantitatively analyzed) and early-passage (3rd) p53MYC (A, C). p53AKT and p53MA showed no detectable amounts of GFAP-specific mRNA in all passages tested (A,B). ** p≤0.01, *** p≤0.001.
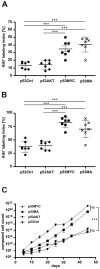
Figure 3. Proliferation kinetics of control and oncogene transduced astrocytes.
The Ki67-LI of the four cell lines at A: the 2nd passage and B: the 7th passage. C: Cells of each cell line were counted at every passage for a period of 45 days. The theoretically derived cell number was computed for each cell line. The p53MYC and p53MA astrocytes showed high proliferation. The p53Ctrl and p53AKT revealed a much lower and comparable growth rate. The mean±SD of three independent experiments is shown. *** p≤0.001.
Figure 4. Expression of c-Myc and Akt induces stem cell marker expression in primary p53−/− astrocytes.
Control cultures (p53Crtl) showed no stem cell marker expression (A I–A IV) and strong GFAP-IR (A V). All oncogene-transduced astrocyte cultures showed a significantly decreased GFAP-IR in early passages (2nd) (B V, D V, F V) and complete loss of GFAP-IR in late (12th) passages (C V, E V, G V) and induced expression of nestin (B II–G II). At late time points (12th passage) expression of Akt additionally induced CD133 (C IV) and Olig2 (CV). Expression of c-Myc led to immunopositive for Musashi-1, nestin, CD133 and Olig2 (E I–IV, G I–IV) and negative for GFAP (E V, G V) (Scale bar: 100 µm).
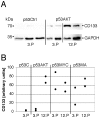
Figure 5. Upregulation of CD133 in transduced astrocytes.
A: Immunoblots for CD133 and GAPDH of protein extracts of control cultures (p53Ctrl) and Akt transduced astrocytes (p53AKT) of the 3rd or 12th passage are shown. B: Normalized band density of CD133 of control cultures (p53C), and transduced astrocytes of the 3rd or 12th passage. In p53AKT and p53MA there is a strong upregulation of CD133 between the 3rd and the 12th passage.
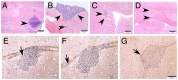
Figure 6. Tumorigenic potential of p53MA astrocytes.
Injection of p53MA astrocytes of a 3rd passage resulted in the development of tumors (arrows) of similar size in C57BL/6 (A) and Rag 2−/− (B) mice, while p53MA astrocytes of an 8th passage led to clearly larger tumors (C). Tumors in C57BL/6 mice revealed only few CD4+ (D, arrow)-, no CD8+ (E) - T-cells and no MAC-1 immunoreactive macrophages (F, scale bar: A–C: 500 µm, D–F: 100 µm).
Figure 7. Tumorsize in wild type and immunocompromized mice.
While 8×104 p53MA cells of an 8th passage produced very large tumors in mice on a C57BL/6 (BL/6) background, 6×104 p53MA astrocytes induced considerably smaller tumors. The comparable size of the tumors in BL/6 and RAG2−/− mice indicated that there was no immune response against the tumor cells in immunocompetent C57BL/6 mice. Tumor volume was measured as described in Materials and Methods.
Similar articles
- Driver or passenger effects of augmented c-Myc and Cdc20 in gliomagenesis.
Ji P, Zhou X, Liu Q, Fuller GN, Phillips LM, Zhang W. Ji P, et al. Oncotarget. 2016 Apr 26;7(17):23521-9. doi: 10.18632/oncotarget.8080. Oncotarget. 2016. PMID: 26993778 Free PMC article. - p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation.
Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, DePinho RA. Zheng H, et al. Nature. 2008 Oct 23;455(7216):1129-33. doi: 10.1038/nature07443. Nature. 2008. PMID: 18948956 Free PMC article. - Extracellular Vesicles Released by Glioblastoma Cells Stimulate Normal Astrocytes to Acquire a Tumor-Supportive Phenotype Via p53 and MYC Signaling Pathways.
Hallal S, Mallawaaratchy DM, Wei H, Ebrahimkhani S, Stringer BW, Day BW, Boyd AW, Guillemin GJ, Buckland ME, Kaufman KL. Hallal S, et al. Mol Neurobiol. 2019 Jun;56(6):4566-4581. doi: 10.1007/s12035-018-1385-1. Epub 2018 Oct 23. Mol Neurobiol. 2019. PMID: 30353492 Free PMC article. - C-Myc Signaling Pathway in Treatment and Prevention of Brain Tumors.
Ashrafizadeh M, Zarabi A, Hushmandi K, Moghadam ER, Hashemi F, Daneshi S, Hashemi F, Tavakol S, Mohammadinejad R, Najafi M, Dudha N, Garg M. Ashrafizadeh M, et al. Curr Cancer Drug Targets. 2021;21(1):2-20. doi: 10.2174/1568009620666201016121005. Curr Cancer Drug Targets. 2021. PMID: 33069197 Review. - Non-neoplastic astrocytes: key players for brain tumor progression.
Catalano M, Limatola C, Trettel F. Catalano M, et al. Front Cell Neurosci. 2024 Jan 16;17:1352130. doi: 10.3389/fncel.2023.1352130. eCollection 2023. Front Cell Neurosci. 2024. PMID: 38293652 Free PMC article. Review.
Cited by
- CDK7 inhibition is a novel therapeutic strategy against GBM both in vitro and in vivo.
Meng W, Wang J, Wang B, Liu F, Li M, Zhao Y, Zhang C, Li Q, Chen J, Zhang L, Tang Y, Ma J. Meng W, et al. Cancer Manag Res. 2018 Nov 15;10:5747-5758. doi: 10.2147/CMAR.S183696. eCollection 2018. Cancer Manag Res. 2018. PMID: 30532595 Free PMC article. - Brain tumor stem cells: Molecular characteristics and their impact on therapy.
Schonberg DL, Lubelski D, Miller TE, Rich JN. Schonberg DL, et al. Mol Aspects Med. 2014 Oct;39:82-101. doi: 10.1016/j.mam.2013.06.004. Epub 2013 Jul 4. Mol Aspects Med. 2014. PMID: 23831316 Free PMC article. Review. - The Microenvironment in Gliomas: Phenotypic Expressions.
Schiffer D, Annovazzi L, Mazzucco M, Mellai M. Schiffer D, et al. Cancers (Basel). 2015 Dec 3;7(4):2352-9. doi: 10.3390/cancers7040896. Cancers (Basel). 2015. PMID: 26633514 Free PMC article. - Modeling and Targeting MYC Genes in Childhood Brain Tumors.
Hutter S, Bolin S, Weishaupt H, Swartling FJ. Hutter S, et al. Genes (Basel). 2017 Mar 23;8(4):107. doi: 10.3390/genes8040107. Genes (Basel). 2017. PMID: 28333115 Free PMC article. Review. - Developmental origins and oncogenic pathways in malignant brain tumors.
Lu QR, Qian L, Zhou X. Lu QR, et al. Wiley Interdiscip Rev Dev Biol. 2019 Jul;8(4):e342. doi: 10.1002/wdev.342. Epub 2019 Apr 3. Wiley Interdiscip Rev Dev Biol. 2019. PMID: 30945456 Free PMC article. Review.
References
- CBTRUS (2012) (2012) Statistical Report: Primary Brain Tumors in the United States, 2004–2008: Central Brain Tumor Registry of the United States, 2012. Available: http://www.cbtrus.org/reports/reports.html.
- Moliterno JA, Patel TR, Piepmeier JM (2012) Neurosurgical approach. Cancer J 18: 20–25. - PubMed
- Weller M, Stupp R, Hegi M, Wick W (2012) Individualized targeted therapy for glioblastoma: fact or fiction? Cancer J 18: 40–44. - PubMed
- Huse JT, Holland EC (2010) Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer 10: 319–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This study was funded by an in-house grant according to the cooperation agreement between the University Hospital (UKGM) and the University of Marburg. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous