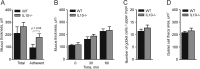Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis - PubMed (original) (raw)
Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis
Malin E V Johansson et al. Gut. 2014 Feb.
Abstract
Objective: The inner mucus layer in mouse colon normally separates bacteria from the epithelium. Do humans have a similar inner mucus layer and are defects in this mucus layer a common denominator for spontaneous colitis in mice models and ulcerative colitis (UC)?
Methods and results: The colon mucus layer from mice deficient in Muc2 mucin, Core 1 O-glycans, Tlr5, interleukin 10 (IL-10) and Slc9a3 (Nhe3) together with that from dextran sodium sulfate-treated mice was immunostained for Muc2, and bacterial localisation in the mucus was analysed. All murine colitis models revealed bacteria in contact with the epithelium. Additional analysis of the less inflamed IL-10(-/-) mice revealed a thicker mucus layer than wild-type, but the properties were different, as the inner mucus layer could be penetrated both by bacteria in vivo and by fluorescent beads the size of bacteria ex vivo. Clear separation between bacteria or fluorescent beads and the epithelium mediated by the inner mucus layer was also evident in normal human sigmoid colon biopsy samples. In contrast, mucus on colon biopsy specimens from patients with UC with acute inflammation was highly penetrable. Most patients with UC in remission had an impenetrable mucus layer similar to that of controls.
Conclusions: Normal human sigmoid colon has an inner mucus layer that is impenetrable to bacteria. The colon mucus in animal models that spontaneously develop colitis and in patients with active UC allows bacteria to penetrate and reach the epithelium. Thus colon mucus properties can be modulated, and this suggests a novel model of UC pathophysiology.
Keywords: BACTERIAL TRANSLOCATION; MUCINS; MUCOSAL BARRIER; MUCOSAL PATHOLOGY; MUCUS.
Figures
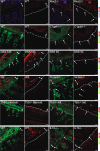
Figure 1
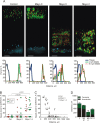
Localisation of bacteria in the inner mucus layer of colon in different mouse colitis models. Fixed colon sections with preserved mucus were immunostained for Muc2 (green) and bacteria as detected by fluorescence in situ hybridisation with general bacterial 16S probes (red) and DNA stained using 4’,6-diamidino-2-phenylindole (DAPI; blue). Wild-type (WT) was compared with the spontaneous colitis models with different disrupted genes (Muc2−/−, C1galT1−/−, Slc9a3−/−, Tlr5−/− and IL-10−/−). Mice were treated with 3% dextran sodium sulfate (DSS) for 12 h or 5 days. Doubled-headed arrows show the inner mucus layer not always free of bacteria. Arrows point to bacteria close to the epithelial cells. Scale bars are 20 µm. Infl., inflamed; Non-infl., non-inflamed.
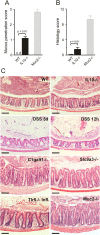
Figure 2
Bacteria penetration and inflammation in different colitis models. (A) Bacteria penetration of the inner mucus was scored for IL-10−/− (n=5) and wild-type (WT; n=5) mice, with Muc2−/−, which have high numbers of bacteria in contact with the epithelium, as comparison (n=3). Data are presented as mean±SEM. Penetration scores for all the genotypes are presented in online
supplementary figure
S1. nd, not detected. (B) Inflammation was monitored as histology scores for IL-10−/− and WT (n=5) mice, with Muc2−/− mice as a severely inflamed comparison (n=3). Data are presented as mean±SEM. Histology scores for all the genotypes are presented in online
supplementary figure
S1. (C) H&E-stained tissue sections corresponding to samples in figure 1. Scale bars in all panels are 100 µm. DSS, 3% dextran sodium sulphate; Infl. inflamed.
Figure 3
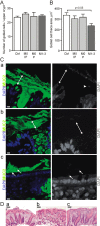
Mucus thickness in wild-type (WT) and interleukin 10-deficient (IL-10−/−) mice. (A) In vivo measurements of the initial mucus thickness in WT (n=7) and IL-10−/− (n=5) mice. The total mucus thickness (Total) was measured followed by aspiration of the mucus and measurement of the remaining mucus thickness (Adherent). (B) Ex vivo measurements of the increase in total mucus thickness over time in WT (n=5) and IL-10−/− (n=6) mice. (C) Number of goblet cells per upper crypt in WT (n=5) and IL-10−/− (n=5) mice. (D) Area of the goblet cell theca in anti-MUC2C3-stained colon section of WT (n=5) and IL-10−/− (n=5) mice. Data are presented as mean±SEM, and the two-tailed Mann–Whitney U test was used to compare the mucus thickness in WT and IL-10−/− mice.
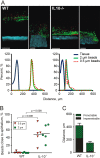
Figure 4
Mucus penetrability in wild-type (WT; n=5) and interleukin 10-deficient (IL-10−/−; n=5) mice. (A) Representative Z-stack projections with the respective normalised intensity plots. Scale bars 100 µm. (B) Percentage of the total bead intensity in close proximity to the epithelial surface (40 µm). Differences between the groups were analysed using a two-tailed Mann–Whitney U test. (C) Relation between penetrable and impenetrable mucus. The black part of the bar represents impenetrable mucus and the green part mucus containing the 2 µm beads (ie, penetrable).
Figure 5
Mucus penetrability in human colonic biopsy samples from controls (n=12) and patients with ulcerative colitis (Mayo 0, n=17, Mayo 1–3, n=11). (A) Representative Z-stack projections with the respective normalised intensity plots. Scale bars 100 µm. (B) Percentage of total bead intensity in close proximity to the epithelial surface (<120 µm). Differences between groups were analysed using the Kruskal–Wallis test with Dunns’ correction for multiple comparisons (primary analysis p=0.0009 for the 2 µm beads and p=0.0004 for the 0.5 µm beads). (C) Percentage of beads in relation to total mucus thickness of individual patients. Numbers refer to patient numbers given in table 1. (D) Relation between penetrable and impenetrable mucus of the 2 µm green beads. C, control; M0 IP, patients with Mayo endoscopic score 0 and impenetrable mucus; M0 P, Mayo score 0 and penetrable mucus (patients number 1, 2 and 12); M1–3, Mayo score 1–3.
Figure 6
Mucus-filled goblet cells and bacteria in fixed human sigmoid biopsy samples. Human colon biopsy samples were Carnoy fixed to preserve the mucus and immunostained for MUC2 in combination with 4’,6-diamidino-2-phenylindole (DAPI) for DNA in nuclei and bacteria. (A) The number of goblet cells per upper crypt was determined in fixed and MU2-stained sections from patients with ulcerative colitis (UC) and controls. (B) The goblet cell theca area was measured in fixed and MU2-stained sections from patients with UC and controls. Data are presented as mean±SEM, and the Kruskal Wallis test with Dunn's correction for multiple comparisons was used to compare the UC patients with the control group. Ctrl, control; M0 IP, patients with endoscopic Mayo score 0 and impenetrable mucus; M0 P, Mayo score 0 and penetrable mucus (patients number 1, 2 and 12); M1–3, Mayo score 1–3. (C) Sections from human sigmoid biopsy samples stained for MUC2 (green) and DAPI (blue). (a) A biopsy specimen collected and directly fixed from sigmoid colon of a control patient without preceding laxative treatment. (b) A biopsy specimen from a control patient included in the penetrability study who was pretreated with laxatives before colonoscopy. (c) Biopsy specimen from patient with UC pretreated with laxative and with a Mayo endoscopic score of 2 at colonoscopy. Pictures to the right only show the DAPI staining. Bacteria (arrows) are found on the outer surface of the mucus in control patients (a and b). Bacteria are found inside the inner mucus and close to the epithelium in the patient with active UC (c). Some detached cells can be observed (arrowhead). (D) H&E-stained tissue each corresponding to parts a–c in (C). Scale bars are 10 µm (C) and 100 µm (D).
Similar articles
- Spontaneous colitis in Muc2-deficient mice reflects clinical and cellular features of active ulcerative colitis.
Wenzel UA, Magnusson MK, Rydström A, Jonstrand C, Hengst J, Johansson ME, Velcich A, Öhman L, Strid H, Sjövall H, Hansson GC, Wick MJ. Wenzel UA, et al. PLoS One. 2014 Jun 19;9(6):e100217. doi: 10.1371/journal.pone.0100217. eCollection 2014. PLoS One. 2014. PMID: 24945909 Free PMC article. - Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model.
Johansson ME, Gustafsson JK, Sjöberg KE, Petersson J, Holm L, Sjövall H, Hansson GC. Johansson ME, et al. PLoS One. 2010 Aug 18;5(8):e12238. doi: 10.1371/journal.pone.0012238. PLoS One. 2010. PMID: 20805871 Free PMC article. - Mucus and the goblet cell.
Johansson ME, Hansson GC. Johansson ME, et al. Dig Dis. 2013;31(3-4):305-9. doi: 10.1159/000354683. Epub 2013 Nov 14. Dig Dis. 2013. PMID: 24246979 Free PMC article. Review. - Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis.
van der Post S, Jabbar KS, Birchenough G, Arike L, Akhtar N, Sjovall H, Johansson MEV, Hansson GC. van der Post S, et al. Gut. 2019 Dec;68(12):2142-2151. doi: 10.1136/gutjnl-2018-317571. Epub 2019 Mar 26. Gut. 2019. PMID: 30914450 Free PMC article. - Ulcerative colitis as a polymicrobial infection characterized by sustained broken mucus barrier.
Chen SJ, Liu XW, Liu JP, Yang XY, Lu FG. Chen SJ, et al. World J Gastroenterol. 2014 Jul 28;20(28):9468-75. doi: 10.3748/wjg.v20.i28.9468. World J Gastroenterol. 2014. PMID: 25071341 Free PMC article. Review.
Cited by
- Mucin-type O-glycans and their roles in intestinal homeostasis.
Bergstrom KS, Xia L. Bergstrom KS, et al. Glycobiology. 2013 Sep;23(9):1026-37. doi: 10.1093/glycob/cwt045. Epub 2013 Jun 10. Glycobiology. 2013. PMID: 23752712 Free PMC article. Review. - The Role of Purported Mucoprotectants in Dealing with Irritable Bowel Syndrome, Functional Diarrhea, and Other Chronic Diarrheal Disorders in Adults.
Alonso-Cotoner C, Abril-Gil M, Albert-Bayo M, Mall JG, Expósito E, González-Castro AM, Lobo B, Santos J. Alonso-Cotoner C, et al. Adv Ther. 2021 May;38(5):2054-2076. doi: 10.1007/s12325-021-01676-z. Epub 2021 Mar 18. Adv Ther. 2021. PMID: 33738725 Free PMC article. Review. - Elevated risk of adverse effects from foodborne contaminants and drugs in inflammatory bowel disease: a review.
Walraven T, Busch M, Wang J, Donkers JM, Duijvestein M, van de Steeg E, Kramer NI, Bouwmeester H. Walraven T, et al. Arch Toxicol. 2024 Nov;98(11):3519-3541. doi: 10.1007/s00204-024-03844-w. Epub 2024 Sep 9. Arch Toxicol. 2024. PMID: 39249550 Free PMC article. Review. - NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion.
Liu C, Xu H, Zhang B, Johansson ME, Li J, Hansson GC, Ghishan FK. Liu C, et al. Am J Physiol Cell Physiol. 2013 Jul 1;305(1):C121-8. doi: 10.1152/ajpcell.00101.2013. Epub 2013 May 8. Am J Physiol Cell Physiol. 2013. PMID: 23657568 Free PMC article. - THE SMALL INTESTINAL MICROBIOME: VIBING WITH INTESTINAL STEM CELLS.
Poplaski V, Sawyer F, Blutt SE. Poplaski V, et al. Microbiota Host. 2023;1(1):e230012. doi: 10.1530/mah-23-0012. Epub 2023 Nov 1. Microbiota Host. 2023. PMID: 38957594 Free PMC article.
References
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34 - PubMed
- Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 2004;126:1620–33 - PubMed
- Danese S, Fiocchi C. Ulcerative Colitis. New Engl J Med 2011;365:1713–25 - PubMed
- Schirbel A, Fiocchi C. Inflammatory bowel disease: established and evolving considerations on its etiopathogenesis and therapy. J Dig Dis 2010;11:266–76 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK099071/DK/NIDDK NIH HHS/United States
- R01 DK083890/DK/NIDDK NIH HHS/United States
- U01AI095473/AI/NIAID NIH HHS/United States
- R01 DK085691/DK/NIDDK NIH HHS/United States
- R01DK073638/DK/NIDDK NIH HHS/United States
- R01 DK073638/DK/NIDDK NIH HHS/United States
- R01 DK041274/DK/NIDDK NIH HHS/United States
- U01 AI095473/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous