Increased activity of the vesicular soluble N-ethylmaleimide-sensitive factor attachment protein receptor TI-VAMP/VAMP7 by tyrosine phosphorylation in the Longin domain - PubMed (original) (raw)
Increased activity of the vesicular soluble N-ethylmaleimide-sensitive factor attachment protein receptor TI-VAMP/VAMP7 by tyrosine phosphorylation in the Longin domain
Andrea Burgo et al. J Biol Chem. 2013.
Abstract
Vesicular (v)- and target (t)-SNAREs play essential roles in intracellular membrane fusion through the formation of cytoplasmic α-helical bundles. Several v-SNAREs have a Longin N-terminal extension that, by promoting a closed conformation, plays an autoinhibitory function and decreases SNARE complex formation and membrane fusion efficiency. The molecular mechanism leading to Longin v-SNARE activation is largely unknown. Here we find that exocytosis mediated by the Longin v-SNARE TI-VAMP/VAMP7 is activated by tonic treatment with insulin and insulin-like growth factor-1 but not by depolarization and intracellular calcium rise. In search of a potential downstream mechanism, we found that TI-VAMP is phosphorylated in vitro by c-Src kinase on tyrosine 45 of the Longin domain. Accordingly, a mutation of tyrosine 45 into glutamate, but not phenylalanine, activates both t-SNARE binding and exocytosis. Activation of TI-VAMP-mediated exocytosis thus relies on tyrosine phosphorylation.
Figures
FIGURE 1.
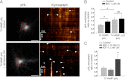
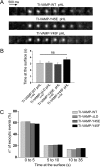
TI-VAMP exocytosis is activated by insulin/IGF-1 cell treatment. A, rat hippocampal neurons were transfected at 2 DIV with TI-VAMP-WT or -ΔLD and treated, or not, overnight with 1 μ
m
insulin (INS). The neurons were then imaged live at 3 DIV, and the exocytic events of TI-VAMP pHL constructs in the cell body (in red boxes) were quantified as described under “Experimental Procedures.” The more relevant exocytic events, here visualized by kymograph (see also
supplemental Movie S1
), appear as bright spots (arrowheads). *, exocytic event not quantified. Scale bars, 20 μm (left panels). B, quantification of exocytic events of TI-VAMP pHL constructs. C, rat hippocampal neurons were transfected at 2 or 7 DIV with TI-VAMP-WT and left untreated or treated for 1 h or overnight with IGF-1. The neurons were then imaged, and exocytic events of TI-VAMP pHL in the cell body were quantified as described above. The data are represented as ratios between the densities per second of exocytic events of TI-VAMP-WT or -ΔLD treated cells and TI-VAMP-WT untreated cells. The data are shown as the means ± S.E. Significance was determined by one-way ANOVA, Dunnett's post test. *, p < 0.05; ns, not significant.
FIGURE 2.
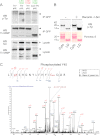
TI-VAMP is phosphorylated by c-Src. A, COS-7 cells were co-transfected with TI-VAMP-WT pHL and Src either in constitutive activated (c-Src Y530F) or inactivated (c-Src Y419F) form. The cells were also transfected with TI-VAMP-WT pHL alone or co-transfected with TI-VAMP-ΔLD pHL and c-Src Y530F as controls. After 16–24 h, the proteins were extracted, and immunoprecipitation (IP) was performed by using monoclonal antibody against GFP. The degree of phosphorylation of TI-VAMP was assessed by using anti-phosphotyrosine antibody 4G10. Circles, TI-VAMP-ΔLD pHL; arrowheads, TI-VAMP-WT pHL; asterisk, c-Src. B, phosphorylation of TI-VAMP in vitro. Recombinant (Recomb.) cytoplasmic domain (Cyto) and Longin domain (LD) of TI-VAMP were phosphorylated in vitro by purified c-Src kinase. Each reaction contained equivalent amounts of Cyto and LD proteins as assessed by Ponceau S Red staining. Src-phosphorylated proteins were detected by Western blotting (WB) using an antibody against phosphotyrosine residues (p-Tyr). C, representative MS/MS spectrum (simultaneous fragmentation of neutral loss product and precursor) for identification of TI-VAMP phosphorylation site and LC electrospray ionization MS/MS spectrum for a carbamidomethylated peptide, with the position of the phosphate group LTpY45SHGNYLFHYICamQDR (756.6(3+) m/z). The fragmentation spectrum derived from trypsin TI-VAMP peptide is shown. The inset shows the peptide sequence and the observed ions obtained from the phospho-peptide. Tandem mass spectrum labeled to show singly and doubly charged b and y ions, as well as ion corresponding to neutral losses of water (circles) and NH3 (asterisks); M, parent ion mass. TIV, TI-VAMP.
FIGURE 3.
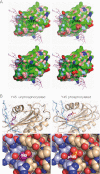
Modeling of Tyr(P)-45 TI-VAMP. A, molecular surface of the TI-VAMP LD (Protein Data Bank code 4B93 (24)). Blue, positively charged atoms; red, negatively charged atoms; green, hydrophobic atoms; salmon, polar oxygens; light blue, polar nitrogens; yellow, sulfur. The SNARE motif (magenta stick model) is shown in its autoinhibitory interaction with LD. The top right panel shows that phosphorylation of Tyr-45 (outlined) adds a bulky charge to the center of a shallow hydrophobic groove. The unphosphorylated molecule is shown for comparison in the top left panel. The bottom panels show homology models for the Y45F and Y45E mutants. B, Tyr-45 (magenta) of the LD (beige) is situated in the binding region for the SNARE motif (light blue). Left panels, Tyr-45 crystal structure 4B93; right panels, Tyr(P)-45 homology model. Top panels, ribbon and stick representation; bottom panels, atoms are shown as van der Waals spheres. Phosphorylation of Tyr-45 introduces a bulky charge in this interaction site, potentially altering ligand interactions (right panels).
FIGURE 4.
The in vitro interaction of TI-VAMP with its molecular partners depends on tyrosine phosphorylation. Quantitative in vitro assay to measure the interaction between recombinant Stx1/SNAP-25 and TI-VAMP mutants. A, COS-7 cells were transfected with GFP alone or GFP-TI-VAMP-WT, -ΔLD, -Y45E, or -Y45F. After 16–24 h, the proteins were extracted and incubated with recombinant t-SNARE complex composed of GST-Stx1 and His6-SNAP-25 immobilized on glutathione beads. TMD, transmembrane domain. B, Coomassie Blue staining of purified recombinant GST-Stx1 and His6-SNAP-25 immobilized on beads. C, Western blots (WB) showing bound GFP fusion proteins eluted from the beads (upper panel) and their expression in the lysates (lower panel). D, quantification of the binding affinity of GFP TI-VAMP (WT and mutated forms) to the recombinant partners. The ratio between each bound GFP fusion protein (pulldown) and its respective expression in the cell lysate is considered. The data are shown as the means ± S.E. Significance was determined by one-way ANOVA, Dunnett's post test. *, p < 0.05; ns, not significant. Circles, GFP TI-VAMP-ΔLD; dashes, GFP; arrowheads, GFP-TI-VAMP-WT, -Y45E, and -Y45F.
FIGURE 5.
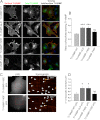
Mutation of Tyr-45 into glutamate but not phenylalanine of the TI-VAMP LD regulates TI-VAMP localization and its mediated exocytosis in COS-7 cells. COS-7 cells were transfected with TI-VAMP-WT, -ΔLD, -Y45E, or -Y45F pHL and processed for surface immunostaining or imaged live after 16–24 h. A, cells were chosen on the basis of total TI-VAMPs pHL expression (green) and processed for quantification of TI-VAMP pHL (red) surface staining. The ratio between red and green staining (overlay) was calculated to evaluate the rate of TI-VAMP exocytosis in the different experimental conditions. Scale bar, 20 μm. B, quantification of the ratio between surface and total expressed TI-VAMP pHL staining (see “Experimental Procedures”). C, exocytosis of TI-VAMP pHL constructs was monitored by fast time lapse video imaging, quantified as described under “Experimental Procedures.” The more relevant exocytic events, here represented by kymograph (see also
supplemental Movie S2
), appear as bright spots (arrowheads). Scale bar, 10 μm (left panels). D, quantification of the density per second of exocytic events for TI-VAMP constructs. The data are shown as the means ± S.E. Significance was determined by one-way ANOVA, Dunnett's post test. *, p < 0.05; ***, p < 0.001; ns, not significant.
FIGURE 6.
TI-VAMP exocytosis kinetics is independent on LD deletion and Tyr-45 mutation. COS-7 cells were transfected with TI-VAMP-WT, -ΔLD, -Y45E, or -Y45F pHL. After 16–24 h, the exocytosis of TI-VAMP pHL constructs was monitored by fast time lapse video imaging. Individual exocytic event appears as diffraction-limited “puffs” of TI-VAMP pHL fluorescence that differed in kinetics. Some TI-VAMP pHLs puffs dissipated quickly (0.5 s), and others remained for a more prolonged time period (>10 s), dissipating gradually over this time period. A, representative snapshots of individual exocytic event for TI-VAMP-WT, -Y45E, and -Y45F pHL with similar kinetics. Scale bars, 500 nm. B, quantification of TI-VAMP pHL construct exocytic kinetics. Kinetics of each individual TI-VAMP pHL puff was quantified by measuring the interval time between its appearance and its dissipation. n, number of individual exocytic event analyzed. ns, not significant. C, quantification of WT and mutant TI-VAMP pHL exocytic kinetics using custom-written scripts in FIJI and Matlab software (see “Experimental Procedures”). Frequency distribution (%) of different temporal classes of TI-VAMP mutants exocytic events (0–5, short; 5–10, intermediate; 10–35, long) are indicated on the abscissa.
FIGURE 7.
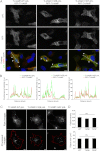
Y45F and Y45E mutations do not alter the subcellular localization, density, and size of TI-VAMP vesicles. A, HeLa cells were co-transfected with TI-VAMP-WT, -Y45E, or -Y45F pHL and RFP-TI-VAMP. TI-VAMP pHL (green) and RFP-TI-VAMP (red) were detected by indirect immunofluorescence using specific GFP and RFP antibodies. Overlay shows co-localization between the different TI-VAMP pHL constructs and RFP-TI-VAMP. Arrowheads point to vesicular structures containing both TI-VAMP pHL and RFP-TI-VAMP. Scale bar, 10 μm. RFP, red fluorescent protein. B, line scans from the dotted lines traced in the overlay images representing the co-localization between TI-VAMP pHL constructs and RFP TI-VAMP. a.u., arbitrary units. C and D, quantification of TI-VAMP membrane structures number (objects density, C) and size (D) per cell. COS-7 cells were transfected with TI-VAMP-WT (n = 18), TI-VAMP-Y45E (n = 18), or TI-VAMP-Y45F (n = 17) pHL and processed for immunofluorescence. The images were analyzed as described under “Experimental Procedures” to obtain the total number of TI-VAMPs GFP positive vesicles per cell (object density) and their size. Significance was determined by one-way ANOVA, Dunnett's post test. ns, not significant.
FIGURE 8.
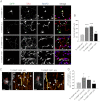
The Y45E substitution increases axonal length and TI-VAMP-mediated exocytosis in hippocampal neurons. Rat hippocampal neurons were transfected at 2 DIV with TI-VAMP-WT, -ΔLD, -Y45E, or -Y45F pHL and processed for immunofluorescence or imaged live after 24 h. A and B, TI-VAMP-Y45E increases axonal length. A, after fixation neurons were labeled for GFP (green), axonal (Tau, red), and dendritic (MAP2, blue) markers. Scale bars, 20 μm. B, quantification of the axonal length (arrowheads) in transfected neurons. C and D, Y45E mutation increases TI-VAMP-mediated exocytosis. Rat hippocampal neurons were transfected with TI-VAMP pHL constructs at 2 DIV and imaged live at 3 DIV. C, exocytic events of TI-VAMP pHL constructs in the cell body (in red boxes) were quantified as described under “Experimental Procedures.” The more relevant exocytic events, here visualized by kymograph (see also
supplemental Movie S3
), appear as bright spots (arrowheads). *, exocytic event not quantified. Scale bars, 20 μm (left panels). D, quantification of exocytic events for TI-VAMP constructs. The data are represented as ratios between the densities per second of exocytic events of TI-VAMP mutants and TI-VAMP-WT and showed as the means ± S.E. Significance was determined by one-way ANOVA, Dunnett's post test. *, p < 0.05; ***, p < 0.001; ns, not significant.
Similar articles
- Alternative splicing of the human gene SYBL1 modulates protein domain architecture of Longin VAMP7/TI-VAMP, showing both non-SNARE and synaptobrevin-like isoforms.
Vacca M, Albania L, Della Ragione F, Carpi A, Rossi V, Strazzullo M, De Franceschi N, Rossetto O, Filippini F, D'Esposito M. Vacca M, et al. BMC Mol Biol. 2011 May 24;12:26. doi: 10.1186/1471-2199-12-26. BMC Mol Biol. 2011. PMID: 21609427 Free PMC article. - Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration.
Proux-Gillardeaux V, Raposo G, Irinopoulou T, Galli T. Proux-Gillardeaux V, et al. Biol Cell. 2007 May;99(5):261-71. doi: 10.1042/BC20060097. Biol Cell. 2007. PMID: 17288539 - A dual mechanism controlling the localization and function of exocytic v-SNAREs.
Martinez-Arca S, Rudge R, Vacca M, Raposo G, Camonis J, Proux-Gillardeaux V, Daviet L, Formstecher E, Hamburger A, Filippini F, D'Esposito M, Galli T. Martinez-Arca S, et al. Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):9011-6. doi: 10.1073/pnas.1431910100. Epub 2003 Jul 9. Proc Natl Acad Sci U S A. 2003. PMID: 12853575 Free PMC article. - Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking.
Chaineau M, Danglot L, Galli T. Chaineau M, et al. FEBS Lett. 2009 Dec 3;583(23):3817-26. doi: 10.1016/j.febslet.2009.10.026. Epub 2009 Oct 20. FEBS Lett. 2009. PMID: 19837067 Review. - Structure and function of longin SNAREs.
Daste F, Galli T, Tareste D. Daste F, et al. J Cell Sci. 2015 Dec 1;128(23):4263-72. doi: 10.1242/jcs.178574. Epub 2015 Nov 13. J Cell Sci. 2015. PMID: 26567219 Review.
Cited by
- Diverse exocytic pathways for mast cell mediators.
Xu H, Bin NR, Sugita S. Xu H, et al. Biochem Soc Trans. 2018 Apr 17;46(2):235-247. doi: 10.1042/BST20170450. Epub 2018 Feb 22. Biochem Soc Trans. 2018. PMID: 29472369 Free PMC article. Review. - The Arabidopsis R-SNARE VAMP721 Interacts with KAT1 and KC1 K+ Channels to Moderate K+ Current at the Plasma Membrane.
Zhang B, Karnik R, Wang Y, Wallmeroth N, Blatt MR, Grefen C. Zhang B, et al. Plant Cell. 2015 Jun;27(6):1697-717. doi: 10.1105/tpc.15.00305. Epub 2015 May 22. Plant Cell. 2015. PMID: 26002867 Free PMC article. - The nonphototrophic hypocotyl 3 (NPH3) domain protein NRL5 is a trafficking-associated GTPase essential for drought resistance.
Upadhyay-Tiwari N, Huang XJ, Lee YC, Singh SK, Hsu CC, Huang SS, Verslues PE. Upadhyay-Tiwari N, et al. Sci Adv. 2024 Aug 9;10(32):eado5429. doi: 10.1126/sciadv.ado5429. Epub 2024 Aug 9. Sci Adv. 2024. PMID: 39121213 Free PMC article. - PTPN9-mediated dephosphorylation of VTI1B promotes ATG16L1 precursor fusion and autophagosome formation.
Chou HY, Lee YT, Lin YJ, Wen JK, Peng WH, Hsieh PL, Lin SY, Hung CC, Chen GC. Chou HY, et al. Autophagy. 2021 Oct;17(10):2750-2765. doi: 10.1080/15548627.2020.1838117. Epub 2020 Oct 28. Autophagy. 2021. PMID: 33112705 Free PMC article. - An interaction network between the SNARE VAMP7 and Rab GTPases within a ciliary membrane-targeting complex.
Kandachar V, Tam BM, Moritz OL, Deretic D. Kandachar V, et al. J Cell Sci. 2018 Dec 10;131(24):jcs222034. doi: 10.1242/jcs.222034. J Cell Sci. 2018. PMID: 30404838 Free PMC article.
References
- Filippini F., Rossi V., Galli T., Budillon A., D'Urso M., D'Esposito M. (2001) Longins. A new evolutionary conserved VAMP family sharing a novel SNARE domain. Trends Biochem. Sci 26, 407–409 - PubMed
- Chaineau M., Danglot L., Galli T. (2009) Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett. 583, 3817–3826 - PubMed
- Burgo A., Proux-Gillardeaux V., Sotirakis E., Bun P., Casano A., Verraes A., Liem R. K., Formstecher E., Coppey-Moisan M., Galli T. (2012) A molecular network for the transport of the TI-VAMP/VAMP7 vesicles from cell center to periphery. Dev. Cell 23, 166–180 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous