Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration - PubMed (original) (raw)
Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration
Zihui Xu et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) share phenotypic and pathologic overlap. Recently, an expansion of GGGGCC repeats in the first intron of C9orf72 was found to be a common cause of both illnesses; however, the molecular pathogenesis of this expanded repeat is unknown. Here we developed both Drosophila and mammalian models of this expanded hexanucleotide repeat and showed that expression of the expanded GGGGCC repeat RNA (rGGGGCC) is sufficient to cause neurodegeneration. We further identified Pur α as the RNA-binding protein of rGGGGCC repeats and discovered that Pur α and rGGGGCC repeats interact in vitro and in vivo in a sequence-specific fashion that is conserved between mammals and Drosophila. Furthermore, overexpression of Pur α in mouse neuronal cells and Drosophila mitigates rGGGGCC repeat-mediated neurodegeneration, and Pur α forms inclusions in the fly eye expressing expanded rGGGGCC repeats, as well as in cerebellum of human carriers of expanded GGGGCC repeats. These data suggest that expanded rGGGGCC repeats could sequester specific RNA-binding protein from their normal functions, ultimately leading to cell death. Taken together, these findings suggest that the expanded rGGGGCC repeats could cause neurodegeneration, and that Pur α may play a role in the pathogenesis of amyotrophic lateral sclerosis and frontotemporal dementia.
Keywords: RNA-mediated neurodegeneration; fly model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
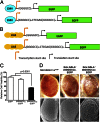
Fig. 1.
Expression of expanded rGGGGCC repeat causes neuronal toxicity in mammalian neuronal cells and Drosophila. (A) Schematic representation of pCMV-(GGGGCC)n-EGFP constructs. Either a 3 GGGGCC repeat or a 30 GGGGCC repeat was inserted upstream of the EGFP coding region, between the indicated transcription and translation start sites. (B) Schematic representation of pUAST-(GGGGCC)n-EGFP constructs. Either a 3 GGGGCC repeat or a 30 GGGGCC repeat was inserted upstream of the EGFP coding region between the indicated transcription and translation start sites. (C) Results of a cell viability assay of Neuro-2a cells transfected with the pEGFP control vector, pCMV-(GGGGCC)3-EGFP, or pCMV-(GGGGCC)30-EGFP. Cells transfected with CMV-(GGGGCC)30-EGFP showed significantly reduced survival (P < 0.0001, t test) compared with cells transfected with either pCMV-(GGGGCC)3-EGFP or pEGFP control. (D) Expression of the rGGGGCC repeat disrupts the Drosophila eye morphology by light (top) and scanning electron (bottom) microscope. All flies are shown at 2 wk after eclosion. (Left) Flies expressing EGFP alone. (Center) Flies expressing (GGGGCC)3-EGFP. (Right) Flies expressing (GGGGCC)30-EGFP.
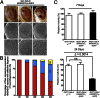
Fig. 2.
Expression of rGGGGCC repeats cause progressive neurodegeneration in the eye and motor neurons of Drosophila. (A) Light microscopy of flies expressing (GGGGCC)30-EGFP, with grade I eye disruption defined as <25% ommatidia loss (_left_), grade II eye disruption defined as 25–50% ommatidia loss with small areas of necrosis (_center_), and grade III eye disruption defined as >50% ommatidia loss with large regions of necrosis (right). SEM images of fly eyes are shown below. (B) Quantification of Drosophila eye disruption on day 1 and week 1, 2, 3 and 4. The eye defects are grouped into three categories: I, II, and III. (C) Effect of expression of rGGGGCC repeats in the fly motor neurons using the OK371-GAL4 driver shown at day 7 (Upper) and day 28 (Lower). Locomotion is given relative to the locomotion observed in the control flies at each time point. Thirty flies were tested in each group. No significant difference was observed at 7 d, but significantly decreased locomotion was observed at 28 d (P = 0.0014, t test) in the flies expressing rGGGGCC30 repeats, but not in flies expressing the rGGGGCC3 repeats.
Fig. 3.
Identification of rGGGGCC RBPs. (A) GGGGCC RNA-binding assays with mouse spinal cord lysates. Biotinylated r(GGGGCC)10 repeat was incubated with increasing concentrations of mouse spinal cord lysates. Lane M refers to the molecular weight marker in all blots. Lane 1, 600 µg of spinal cord lysate only; lane 2, 300 µM biotin incubated with 600 µg of spinal cord lysate; lanes 3–8, 300 µM biotinylated r(GGGGCC)10 repeat incubated with 30, 60, 150, 300, 450, and 600 µg of spinal cord lysate. (B) rGGGGCC repeat RBP competition assay with excess unlabeled r(GGGGCC)10 repeat. Lane 1, 300 µM biotinylated r(GGGGCC)10 repeat only; lane 2, 300 µM biotinylated r(GGGGCC)10 repeat and 10× r(GGGGCC)10 repeat; lane 3, 300 µM biotinylated r(GGGGCC)10 repeat and 100× r(GGGGCC)10 repeat. All lanes were incubated with 300 µg of spinal cord lysate. (C) rGGGGCC repeat RBP competition assay with excess unlabeled r(CGG)10 repeat. Lane 1, 300 µM biotinylated r(GGGGCC)10 repeat only; lane 2, 300 µM biotinylated r(GGGGCC)10 repeat and 10× r(CGG)10 repeat; lane 3, 300 µM biotinylated r(GGGGCC)10 repeat and 100× r(CGG)10 repeat. All lanes were incubated with 300 µg of spinal cord lysate. (D) Work flow schematic for identification of RBPs by MS.
Fig. 4.
Pur α binds rGGGGCC repeats in a dose-dependent manner in vitro and in vivo. (A) rGGGGCC binding assay with mouse and Drosophila Pur α and mouse hnRNP A2/B1. 32P-labeled r(GGGGCC)10 was incubated with increasing concentrations of recombinant Pur α (mouse), Pur α (Drosophila), hnRNP A2/B1, and GST alone. (B) In vivo interaction of rGGGGCC repeats and Pur α. Neuro-2a cells were cotransfected with the FLAG-tagged Pur α and either EGPF, (GGGGCC)3-EGFP, or (GGGGCC)30-EGFP constructs, and immunoprecipitation was performed with either mouse IgG or anti–FLAG-M2 antibodies. (Upper) Western blots of precipitate using IgG control and anti–FLAG-M2 antibodies. The input lane is a Neuro-2a cell expressing FLAG-tagged Pur α alone. (Lower) Results of qRT-PCR for EGFP RNA for each precipitate. (C) Pur α binds rGGGGCC from mouse or human brain lysate. Biotinylated (GGGGCC)10 repeats were incubated with brain lysate. Lanes 1 and 5, 30 µg of brain lysate; lanes 2 and 6, 300 µg of brain lysate; lanes 3 and 7, 300 µg of brain lysate incubated with 300 µM biotin; lanes 4 and 8, 300 µg brain lysate incubated with 300 µM biotinylated r(GGGGCC)10 repeat.
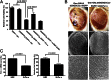
Fig. 5.
Overexpression of Pur α suppresses rGGGGCC repeat-mediated neurodegeneration. (A) Pur α overexpression attenuates rGGGGCC repeat-mediated cell death in Neuro-2a cells. Neuro-2a cells were cotransfected using GGGGCC repeat-expressing constructs and Pur α or hnRNP A2/B1 expression vectors. A significant reduction in cell viability was seen for Neuro-2a cells transfected with the (GGGGCC)30 construct and pcDNA control vector compared with cells transfected with the (GGGGCC)3 construct or control EGFP expression vector (P < 0.0001, t test). Pur α overexpression in the presence of (GGGGCC)30 construct resulted in significantly greater cell viability (P = 0.0041, t test). (B) Light (top) and electron (middle and bottom) microscopy of flies overexpressing Pur α and rGGGGCC repeats in the fly eye. (Left) Flies expressing (GGGGCC)30-EGFP. (Right) Flies expressing (GGGGCC)30-EGFP and Pur α. (C) Knockdown of Pur α induces cell death in Neuro-2a cells. Transient transfection of siRNA against Pur α significantly reduced Pur α mRNA levels (Left) (P < 0.0001, t test), as detected by qRT-PCR, and cell viability (Right) (P = 0.0022, t test).
Fig. 6.
Pur α inclusions in rGGGGCC Drosophila and human GGGGCC expansion repeat carriers. (A) Drosophila Pur α and ubiquitin colocalize in rGGGGCC-induced inclusions in flies expressing expanded rGGGGCC repeats. Confocal images are shown of eye transverse sections from 14-d-old flies with either rGGGGCC3 (control) or GGGGCC30 in trans to gmr-GAL4, stained with antibodies against ubiquitin (red) and Pur α (green). The nuclei were stained with DAPI (blue). (B) Pur α forms inclusions in the cerebellum of humans with FTLD-TDP. Molecular and granule cell layers of the cerebellum in individuals with FTLD-TDP with expanded rGGGGCC repeats are shown stained for p62 and Pur α. In the molecular layer, p62-positive TDP-negative intranuclear inclusions are shown as red arrowheads, and Pur α-staining intranuclear inclusions are shown as blue arrowheads. In the granule cell layer, p62-positive TDP-negative inclusions are shown as green arrowheads, and Pur α inclusions are shown as black arrowheads.
Comment in
- Toxic RNA as a driver of disease in a common form of ALS and dementia.
Orr HT. Orr HT. Proc Natl Acad Sci U S A. 2013 May 7;110(19):7533-4. doi: 10.1073/pnas.1305239110. Epub 2013 Apr 29. Proc Natl Acad Sci U S A. 2013. PMID: 23630297 Free PMC article. No abstract available.
Similar articles
- The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions.
Simón-Sánchez J, Dopper EG, Cohn-Hokke PE, Hukema RK, Nicolaou N, Seelaar H, de Graaf JR, de Koning I, van Schoor NM, Deeg DJ, Smits M, Raaphorst J, van den Berg LH, Schelhaas HJ, De Die-Smulders CE, Majoor-Krakauer D, Rozemuller AJ, Willemsen R, Pijnenburg YA, Heutink P, van Swieten JC. Simón-Sánchez J, et al. Brain. 2012 Mar;135(Pt 3):723-35. doi: 10.1093/brain/awr353. Epub 2012 Feb 1. Brain. 2012. PMID: 22300876 - Transcription elongation factor AFF2/FMR2 regulates expression of expanded GGGGCC repeat-containing C9ORF72 allele in ALS/FTD.
Yuva-Aydemir Y, Almeida S, Krishnan G, Gendron TF, Gao FB. Yuva-Aydemir Y, et al. Nat Commun. 2019 Nov 29;10(1):5466. doi: 10.1038/s41467-019-13477-8. Nat Commun. 2019. PMID: 31784536 Free PMC article. - Purα Repaired Expanded Hexanucleotide GGGGCC Repeat Noncoding RNA-Caused Neuronal Toxicity in Neuro-2a Cells.
Shen J, Zhang Y, Zhao S, Mao H, Wang Z, Li H, Xu Z. Shen J, et al. Neurotox Res. 2018 May;33(4):693-701. doi: 10.1007/s12640-017-9803-0. Epub 2017 Oct 3. Neurotox Res. 2018. PMID: 28975482 - Pathogenic determinants and mechanisms of ALS/FTD linked to hexanucleotide repeat expansions in the C9orf72 gene.
Wen X, Westergard T, Pasinelli P, Trotti D. Wen X, et al. Neurosci Lett. 2017 Jan 1;636:16-26. doi: 10.1016/j.neulet.2016.09.007. Epub 2016 Sep 13. Neurosci Lett. 2017. PMID: 27619540 Free PMC article. Review. - Molecular Mechanisms of Neurodegeneration Related to C9orf72 Hexanucleotide Repeat Expansion.
Babić Leko M, Župunski V, Kirincich J, Smilović D, Hortobágyi T, Hof PR, Šimić G. Babić Leko M, et al. Behav Neurol. 2019 Jan 15;2019:2909168. doi: 10.1155/2019/2909168. eCollection 2019. Behav Neurol. 2019. PMID: 30774737 Free PMC article. Review.
Cited by
- Toxic RNA as a driver of disease in a common form of ALS and dementia.
Orr HT. Orr HT. Proc Natl Acad Sci U S A. 2013 May 7;110(19):7533-4. doi: 10.1073/pnas.1305239110. Epub 2013 Apr 29. Proc Natl Acad Sci U S A. 2013. PMID: 23630297 Free PMC article. No abstract available. - RNautophagy/DNautophagy possesses selectivity for RNA/DNA substrates.
Hase K, Fujiwara Y, Kikuchi H, Aizawa S, Hakuno F, Takahashi S, Wada K, Kabuta T. Hase K, et al. Nucleic Acids Res. 2015 Jul 27;43(13):6439-49. doi: 10.1093/nar/gkv579. Epub 2015 Jun 1. Nucleic Acids Res. 2015. PMID: 26038313 Free PMC article. - Optogenetic control of GGGGCC repeat-containing RNA phase transition.
Li X, Lu S, Lu B, Sun X. Li X, et al. Fundam Res. 2022 Sep 9;2(6):843-850. doi: 10.1016/j.fmre.2022.09.001. eCollection 2022 Nov. Fundam Res. 2022. PMID: 38933387 Free PMC article. - Gene Therapy for Neurodegenerative Diseases: Slowing Down the Ticking Clock.
Martier R, Konstantinova P. Martier R, et al. Front Neurosci. 2020 Sep 18;14:580179. doi: 10.3389/fnins.2020.580179. eCollection 2020. Front Neurosci. 2020. PMID: 33071748 Free PMC article. Review. - RNA Structures as Mediators of Neurological Diseases and as Drug Targets.
Bernat V, Disney MD. Bernat V, et al. Neuron. 2015 Jul 1;87(1):28-46. doi: 10.1016/j.neuron.2015.06.012. Neuron. 2015. PMID: 26139368 Free PMC article. Review.
References
- Fecto F, Siddique T. Making connections: Pathology and genetics link amyotrophic lateral sclerosis with frontotemporal lobe dementia. J Mol Neurosci. 2011;45(3):663–675. - PubMed
- Ringholz GM, et al. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65(4):586–590. - PubMed
- Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59(7):1077–1079. - PubMed
- Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. - PubMed
- Mackenzie IR, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61(5):427–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS051630/NS/NINDS NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- R01 NS051630/NS/NINDS NIH HHS/United States
- P50AG025688/AG/NIA NIH HHS/United States
- R21 NS067461/NS/NINDS NIH HHS/United States
- P30 NS055077/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous