Single-cell analysis and sorting using droplet-based microfluidics - PubMed (original) (raw)
Single-cell analysis and sorting using droplet-based microfluidics
Linas Mazutis et al. Nat Protoc. 2013 May.
Abstract
We present a droplet-based microfluidics protocol for high-throughput analysis and sorting of single cells. Compartmentalization of single cells in droplets enables the analysis of proteins released from or secreted by cells, thereby overcoming one of the major limitations of traditional flow cytometry and fluorescence-activated cell sorting. As an example of this approach, we detail a binding assay for detecting antibodies secreted from single mouse hybridoma cells. Secreted antibodies are detected after only 15 min by co-compartmentalizing single mouse hybridoma cells, a fluorescent probe and single beads coated with anti-mouse IgG antibodies in 50-pl droplets. The beads capture the secreted antibodies and, when the captured antibodies bind to the probe, the fluorescence becomes localized on the beads, generating a clearly distinguishable fluorescence signal that enables droplet sorting at ∼200 Hz as well as cell enrichment. The microfluidic system described is easily adapted for screening other intracellular, cell-surface or secreted proteins and for quantifying catalytic or regulatory activities. In order to screen ∼1 million cells, the microfluidic operations require 2-6 h; the entire process, including preparation of microfluidic devices and mammalian cells, requires 5-7 d.
Figures
Figure 1
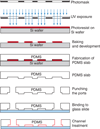
Schematic of soft lithography.
Figure 2
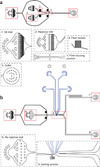
Design of microfluidic devices. (a) Microfluidic device for cell and bead coencapsulation. The device consists of one inlet for the continuous phase (1) and two inlets for different aqueous phases (2). Fluid resistors (3) are placed for both continuous and aqueous phases to dampen fluctuations arising from the mechanical instability of syringe pumps and the elasticity of the PDMS device. Because of laminar flow inside microfluidic channels, the two aqueous streams injected into the device flow side by side and are completely mixed only after they are encapsulated into droplets at the flow-focusing junction (4). Droplets leaving the outlet (5) are collected into a tube or syringe. (b) The droplet-sorting device consists of one inlet for the continuous phase (1), one inlet for the re-injected emulsion (6); and two outlets, one for sorted droplets (7) and the other for unsorted droplets (8). After spacing the emulsion droplets with carrier oil, they move down to the sorting junction (9), where individual droplets are sorted on the basis of fluorescence intensity. The edges of microfluidic channels are black, the edges of electrodes are blue, and the red triangle is a mark below the microfluidic channel to indicate where the laser beam should be focused and droplet fluorescence measured. See also supplementary Videos 1–3 and supplementary Data.
Figure 3
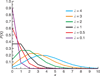
Probability P(X=x) of finding x number of entities (cell, bead, etc.) at different λ values.
Figure 4
Principle of experimental design. Two types of cells (orange and gray ellipses) are introduced together as a cell suspension into a microfluidic device together with a bead suspension. One cell type produces mouse antibodies (gray) and the other does not (orange), with the latter being in 10-fold excess. The bead suspension contains green fluorescent–labeled goat detection antibodies (green) and streptavidin beads coated with goat anti-mouse-Fc capture antibodies (magenta). Because of laminar flow, the two suspensions are only mixed inside the droplets, which are created by flow focusing with fluorinated oil containing fluorosurfactant and then collected off-chip at 4 °C. After incubation for 15 min at 37 °C and 5% CO2, those beads that are coencapsulated with an antibody-producing cell become highly fluorescent, due to the capture of secreted antibodies on the bead by the anti-mouse Fc antibody and binding of the green fluorescence detection antibodies to the captured antibodies in a sandwich assay. The emulsion is then introduced into a second microfluidic device and droplets containing green fluorescent beads are sorted using a fluorescence-activated droplet sorter. Hence, droplets containing no bead, no cell, a cell which does not secrete antibody, or an antibody-producing cell but no bead are discarded (no green fluorescent bead is present), whereas droplets containing an antibody-producing cell and a bead (which becomes fluorescent) are collected. The three micrographs show coencapsulation of cells with beads (left), droplet re-injection after incubation off-chip (middle) and droplet sorting (right). Ab, antibody.
Figure 5
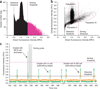
Fluorescence analysis and sorting of droplets. A mixture of two types of cells, 9E10 (mouse hybridoma cells that secrete anti-MYC antibody) and K562 (human leukemia cells that do not produce antibodies) were coencapsulated with capture beads into 50 pl droplets. K562 cells, which were in 10-fold excess with respect to 9E10 cells, were prestained with orange CMRA dye before encapsulation making them distinguishable from 9E10 cells. (a) Histogram showing the distribution of droplet green fluorescence intensity. The detection threshold (black dashed line) was set at 0.5 relative fluorescence units (RFU) to detect all the droplets passing through the detection point. Fluorescent droplets passing the sorting threshold (magenta dashed line) were sorted and collected off-chip into a tube. The droplet population having green fluorescence at ~1.0 RFU corresponds to droplets having cells that do not produce antibodies, as well as droplets with no cells. (b) 2D scatter plot of droplet fluorescence intensity during the sorting process. Purple-dashed ellipses indicate different droplet populations. Population I contains droplets with a K562 cell (with or without a bead). Population II contains droplets with a 9E10 cell and no bead, and droplets lacking any cell (with or without a bead). Population III contains droplets with a 9E10 cell and a bead. Droplets containing K562 and 9E10 cells coencapsulated with a bead fall into the region between ~0.5 and 1.0 orange RFU and ~1.2 and 3.0 green RFU. The slight tilt observed in green fluorescence is due to the small fraction of green fluorescence being detected by the orange PMT. (c) Fluorescence intensity of individual droplets during microfluidic analysis and sorting. See also supplementary Video 3.
Figure 6
Schematic of the optical setup. The fluorescence of each droplet is measured as the droplet flows past an observation constraint (OC) in the microfluidic channel in which the whole droplet is constrained to have a cross-section area of 20 × 25 µm as it passes an optical measurement fiduciary mark on the chip. In this OC, a 50-pl droplet will extend to be ~100 µm in length. The fluorescence measurement setup uses a 50-mW, 488-nm laser and a Nikon Diaphot epi-illumination microscope. Between the laser and the epi-illumination port of the microscope are ‘shaping optics’ whose task is to form the beam to a narrow line and present it at the input epi-image plane of the Diaphot’s 160-mm tube objectives. A Thorlabs OSL1 visible light illuminator is mounted through a 600-nm long-pass filter to provide optional transillumination to the sample and allow simultaneous trans and epi work if needed. The microscope has a side port, where we place the optics for three PMTs to detect three different colors. The microscope contains an internal prism that we use to send 80% of the light collected by its objectives to the PMTs, whereas 20% is routed to the microscope eyepieces. This 80/20 split enables us to mount a digital high-speed camera in one eyepiece to do simultaneous transmission imaging video and three-color-PMT–based fluorescence measurements.
Figure 7
Compartmentalized single hybridoma cells before and after microfluidic sorting. (a–f) The upper row shows micrographs of droplets after incubation at 37 °C for 15 min and the lower row shows droplets after microfluidic sorting. (a,d) Bright-field images. (b,e) Orange fluorescence, which is due to the presence of K562 cells. (c,f) Green fluorescence, due to 9E10-secreted antibodies binding to the beads. The green fluorescence signal on the beads is the result of 9E10-secreted antibodies captured on the beads by an anti-mouse Fc antibody and then in turn bound by a green fluorescence–labeled goat anti-mouse-F(ab)2 antibody. These binding events result in the green fluorescent anti-mouse-F(ab)2 antibody becoming concentrated on the beads instead of being distributed homogeneously throughout the volume of the droplet. The assay is therefore similar in nature to a sandwich ELISA, but it relies on the detection of the bead-bound detection antibody, rather than the measurement of detection antibodies after washing, as in ELISA. Note that in d, some beads are not clearly visible, as they occupy a different focal plane. The pink dashed box shows a droplet containing a 9E10 cell and a green fluorescent bead. Scale bars, 50 µm.
Similar articles
- Single-Cell Droplet Microfluidic Screening for Antibodies Specifically Binding to Target Cells.
Shembekar N, Hu H, Eustace D, Merten CA. Shembekar N, et al. Cell Rep. 2018 Feb 20;22(8):2206-2215. doi: 10.1016/j.celrep.2018.01.071. Cell Rep. 2018. PMID: 29466744 Free PMC article. - An Automated Single-Cell Droplet-Digital Microfluidic Platform for Monoclonal Antibody Discovery.
Ahmadi F, Tran H, Letourneau N, Little SR, Fortin A, Moraitis AN, Shih SCC. Ahmadi F, et al. Small. 2024 Jun;20(26):e2308950. doi: 10.1002/smll.202308950. Epub 2024 Mar 5. Small. 2024. PMID: 38441226 - Encapsulation of single cells on a microfluidic device integrating droplet generation with fluorescence-activated droplet sorting.
Wu L, Chen P, Dong Y, Feng X, Liu BF. Wu L, et al. Biomed Microdevices. 2013 Jun;15(3):553-60. doi: 10.1007/s10544-013-9754-z. Biomed Microdevices. 2013. PMID: 23404263 - Design and construction of a microfluidics workstation for high-throughput multi-wavelength fluorescence and transmittance activated droplet analysis and sorting.
Panwar J, Autour A, Merten CA. Panwar J, et al. Nat Protoc. 2023 Apr;18(4):1090-1136. doi: 10.1038/s41596-022-00796-2. Epub 2023 Jan 27. Nat Protoc. 2023. PMID: 36707723 Review. - Development of Droplet Microfluidics Enabling High-Throughput Single-Cell Analysis.
Wen N, Zhao Z, Fan B, Chen D, Men D, Wang J, Chen J. Wen N, et al. Molecules. 2016 Jul 5;21(7):881. doi: 10.3390/molecules21070881. Molecules. 2016. PMID: 27399651 Free PMC article. Review.
Cited by
- High-throughput screening for optimizing adoptive T cell therapies.
Zhang Y, Xu Q, Gao Z, Zhang H, Xie X, Li M. Zhang Y, et al. Exp Hematol Oncol. 2024 Nov 13;13(1):113. doi: 10.1186/s40164-024-00580-w. Exp Hematol Oncol. 2024. PMID: 39538305 Free PMC article. Review. - NOVAsort for error-free droplet microfluidics.
Zhang H, Gupte R, Li Y, Huang C, Guzman AR, Han JJ, Jung H, Sabnis R, de Figueiredo P, Han A. Zhang H, et al. Nat Commun. 2024 Nov 1;15(1):9444. doi: 10.1038/s41467-024-52932-z. Nat Commun. 2024. PMID: 39487108 Free PMC article. - Tuning porosity of macroporous hydrogels enables rapid rates of stress relaxation and promotes cell expansion and migration.
Nerger BA, Kashyap K, Deveney BT, Lou J, Hanan BF, Liu Q, Khalil A, Lungjangwa T, Cheriyan M, Gupta A, Jaenisch R, Weitz DA, Mahadevan L, Mooney DJ. Nerger BA, et al. Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2410806121. doi: 10.1073/pnas.2410806121. Epub 2024 Oct 28. Proc Natl Acad Sci U S A. 2024. PMID: 39467139 - Stimulated Brillouin scattering flow cytometry.
Rosvold JR, Zanini G, Handler C, Frank E, Li J, Vitolo MI, Martin SS, Scarcelli G. Rosvold JR, et al. Biomed Opt Express. 2024 Sep 25;15(10):6024-6035. doi: 10.1364/BOE.537602. eCollection 2024 Oct 1. Biomed Opt Express. 2024. PMID: 39421786 Free PMC article. - Single-Cell Microfluidics: A Primer for Microbiologists.
Ripandelli RAA, van Oijen AM, Robinson A. Ripandelli RAA, et al. J Phys Chem B. 2024 Oct 24;128(42):10311-10328. doi: 10.1021/acs.jpcb.4c02746. Epub 2024 Oct 14. J Phys Chem B. 2024. PMID: 39400277 Free PMC article. Review.
References
- Guo MT, Rotem A, Heyman JA, Weitz DA. Droplet microfluidics for high-throughput biological assays. Lab Chip. 2012;12:2146–2155. - PubMed
- Kintses B, van Vliet LD, Devenish SR, Hollfielder F. microfluidic droplets: new integrated workflows for biological experiments. Curr. Opin. Chem. Biol. 2010;14:548–555. - PubMed
- Theberge AB, et al. Microdroplets in microfluidics: an evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed. 2010;49:5846–5868. - PubMed
- Dove A. Drug screening--beyond the bottleneck. Nat. Biotechnol. 1999;17:859–863. - PubMed
- Link DR, Anna SL, Weitz DA, Stone HA. Geometrically mediated breakup of drops in microfluidic devices. Phys. Rev. Lett. 2004;92:054503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5R01EB014703-02/EB/NIBIB NIH HHS/United States
- P01 GM096971/GM/NIGMS NIH HHS/United States
- 1R43AI082861-01/AI/NIAID NIH HHS/United States
- R01 EB014703/EB/NIBIB NIH HHS/United States
- P01GM096971/GM/NIGMS NIH HHS/United States
- R43 AI082861/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials