Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORγt and Ahr that leads to IL-17 production by activated B cells - PubMed (original) (raw)
doi: 10.1038/ni.2569. Epub 2013 Apr 7.
Shaun W Jackson, Melisa Gorosito-Serran, Eva V Acosta-Rodriguez, Maria C Amezcua-Vesely, Blythe D Sather, Akhilesh K Singh, Socheath Khim, Juan Mucci, Denny Liggitt, Oscar Campetella, Mohamed Oukka, Adriana Gruppi, David J Rawlings
Affiliations
- PMID: 23563688
- PMCID: PMC3631452
- DOI: 10.1038/ni.2569
Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORγt and Ahr that leads to IL-17 production by activated B cells
Daniela A Bermejo et al. Nat Immunol. 2013 May.
Abstract
Here we identified B cells as a major source of rapid, innate-like production of interleukin 17 (IL-17) in vivo in response to infection with Trypanosoma cruzi. IL-17(+) B cells had a plasmablast phenotype, outnumbered cells of the TH17 subset of helper T cells and were required for an optimal response to this pathogen. With both mouse and human primary B cells, we found that exposure to parasite-derived trans-sialidase in vitro was sufficient to trigger modification of the cell-surface mucin CD45, which led to signaling dependent on the kinase Btk and production of IL-17A or IL-17F via a transcriptional program independent of the transcription factors RORγt and Ahr. Our combined data suggest that the generation of IL-17(+) B cells may be a previously unappreciated feature of innate immune responses required for pathogen control or IL-17-mediated autoimmunity.
Figures
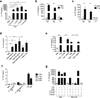
Figure 1. B cells from T. cruzi infected mice produce IL-17
(a) Representative flow cytometry plots showing IL-17A expression in B220+ cells in the spleen of wild-type (WT) and µMT mice infected with T. cruzi at 10 days (d) post-infection. (b) Number of IL-17A-expressing splenic CD4+, CD8+ and B220+ cells in uninfected (UI), or 10 and 19 d _T. cruzi_-infected mice (WT or µMT). Each symbol denotes a single animal and the line represents the mean value in each group (6–9 animals per group). Data are representative of 4 independent experiments. (c) IL-17A expression (at 10 d) in total splenocytes from _T. cruzi_-infected WT mice (left) and in gated CD19+ cells expressing the plasma cell marker, CD138, or the GC B cell marker, GL7 (right). Data are representative of 3 experiments. (d) Immunofluorescence staining of splenic sections from UI and T. cruzi infected (10 d) mice showing IL-17A and IgM expression (magenta and cyan, respectively, left; and merged images, right). Arrow indicates IL-17+IgM+ cells. Dashed lines surround less strongly staining (e.g. IgMlo) B cell follicles (*). Data are representative of 3 experiments. (e) IL-17A mRNA expression in total, sorted B220+ and B220− splenocytes from infected mice cultured in media alone or with PMA and ionomycin (PMA+Iono). HPRT was used for normalization (10 d, 3 replicates per condition). (f) IL-17A production by B220+ splenocytes from UI or infected (Inf) mice cultured with medium or PMA+Iono for 72 h (10 d, 3 replicates per condition). Data (e–f) are representative of 2 experiments. (g) IL-17A+ B220+ cells in _T. cruzi_-infected µMT or B-cell reconstituted µMT (µMT + B cells) mice (10 d). Lower panel shows IL-17A expression predominantly within the B220+CD138+ gate. Data are representative of 3 experiments. (h) Number of IL-17+ B220+ cells within the spleen of UI or Inf WT, µMT or B cell reconstituted µMT mice (10 d; 6–7 mice per condition). Data are representative of 3 experiments. All error bars denote s.e.m. *, P ≤ 0.05 **, P ≤ 0.005 (calculated by Mann-Whitney _U_-test).
Figure 2. B cell IL-17A production is required for T. cruzi infection control
Control WT, µMT, or µMT mice reconstituted with WT or Il17a−/− B cells, were infected with T. cruzi and evaluated for: (a) Parasitemia (left panel: kinetic; right panels: day 10 and 15); (b) Survival based on Kaplan-Meier survival curve; (c) Serum IFNγ and TNF concentrations; and, (e) plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activity. Surviving animals were sacrificed at 20 d and (d) IFN-γ and TNF production by unstimulated splenocytes was quantified (72 hour in vitro culture). Each symbol denotes a single animal and the line represents the mean value in each group. All error bars denote s.e.m. *, P ≤ 0.05 **, P ≤ 0.005 (calculated by Mann-Whitney _U_-test). Results shown are from 2 independent experiments with 3–4 animals per group in each experiment.
Figure 3. Exposure to T. cruzi trypomastigotes or purified trans-sialidase is sufficient to trigger B cell IL-17 production
(a,d–g) IL-17A secretion by B220+CD19+ cells or (b–c) IL-17A and IL-17F production by follicular (FM), marginal zone (MZ) and transitional 1 (T1) B cell subsets purified from spleens of UI WT mice and stimulated for 72 h with: (a) various doses of live T. cruzi trypomastigotes (Tryp) or T. cruzi antigen (Ag); or (b–g) 1 × 106 Tryp or different doses of active vs. inactive trans-sialidase (Tsial) with or without a Tsial-neutralizing mAb (α-Tsial) or IgG2a isotype control. (f) B220+CD19+ cells from UI WT mice were cultured with Tryp, Tsial or different doses of bacterial V. cholerae and C. perfringens neuramidases (NA). (g) B220+CD19+ cells from WT mice were cultured with Tryp or Tsial for 72 h in medium containing FBS or 2% BSA with alpha(2,3)-sialyllactose (α-2,3-SL) and alpha(2,6)-sialyllactose (α-2,6-SL) or in identical media lacking acceptor lactose. All error bars denote s.e.m. *, P ≤ 0.05 **, P ≤ 0.005 (calculated by Mann-Whitney _U_-test). Results are representative of 3 independent experiments with 2 replicates per condition. ND: None detectable.
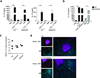
Figure 4. CD45 and Btk are required for B cell IL-17 production in response to T. cruzi trypomastigotes or purified trans-sialidase
(a) IL-17A production in follicular B cells from WT mice in response to T. cruzi Tryp and treated with or without various doses of CD45 inhibitor. Similar analysis was performed using B cells isolated from Cd45−/− mice. (b) IL-17A expression in CD19+ splenic cells from 10 d infected µMT mice that were recipients of adoptively transferred WT (left) or Cd45−/− B cells (right). (c) Number of CD19+IL-17A+ cells in spleen of UI vs. 10 d infected animals including: WT or µMT mice, or µMT recipients of WT vs. Cd45−/− B cells. Each symbol denotes a mouse and the line represents the median value in each group. (d) In vitro IL-17A secretion by B220+CD19+ cells derived from WT vs. Gal-1−/−Gal-3−/− mice in response to Tryp-stimulation. (e) IL-17A production by WT FM B cells stimulated with Tryp and treated with different concentration of Src inhibitor (PP2) vs. DMSO control. (f) IL-17A production by WT FM B cells stimulated with Tryp and treated with increasing doses of Btk-specific inhibitors (AVL-292 or CNX-652) vs. DMSO control. IL-17A production by Tryp stimulated FO B cells derived from Btk−/− and Btk−/−Tec−/− mice is also shown. (g) Representative FACS plots (left panels) and total number of splenic CD19+IL-17A+ cells (right panel) in T. cruzi infected (10 d) WT or Btk−/− mice. (h) IL17A production in Tryp stimulated purified B cells derived from BALB/c (control) vs. Pkcβ−/− mice. All in vitro analyses were performed at 72 h and results are representative of three (a–c) or two (d–g) independent experiments for each strain or condition. Data are shown as means ± s.e.m. *, P ≤ 0.05 **, P ≤ 0.005 (calculated by one-way ANOVA with Bonferroni correction (a–f, h) and Mann-Whitney _U_-test (g)).
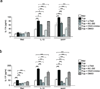
Figure 5. RORγt and AHR are not required for B cell IL-17 production in response to T. cruzi
(a) IL-17 secretion by WT and Rorγt−/− splenic B cells cultured in vitro for 72 hr with T. cruzi Tryp, with or without Tsial-neutralizing mAb (α-Tsial) (4 replicates per condition). (b) IL-17A production in non-stimulated and Tryp-stimulated, WT B cells (in presence or absence of the RORγt and RORα inhibitor SR1001, 25µM) and Ahr−/− B cells. Data are shown as means ± s.e.m. *, P ≤ 0.05 **, P ≤ 0.005 (calculated by one-way ANOVA with Bonferroni correction). (c) Number of splenic B220+IL-17A+ cells in T. cruzi infected (10 d) WT, Rorγt−/− and Ahr−/− mice. Each symbol denotes a mouse and the line represents the median value in each group. (d) Rorγt locus activation cannot be detected in vivo in B cells following T. cruzi infection. RORγt-cre × ROSA-RFP reporter mice were infected with T. cruzi and spleens collected at 10 d post-infection. ROSA-RFP (magenta) and IgM (cyan) expression in spleen sections from UI (upper panels) vs. 10 d-infected (lower panels) animals. Results are representative of three (a), two (b, c), or one (d) experiment for each strain or condition.
Figure 6. Primary human B cells stimulated with T. cruzi produce IL-17
Purified human CD19+ tonsillar B cells were cultured for 72 hr in presence of recombinant IL-10 or BAFF and incubated with T. cruzi Tryp with or without α-Tsial mAb. B cells were also treated with Btk (AVL-292) or CD45 inhibitors or DMSO control. Production of (a) IL-17A or (b) IL-17F determined by ELISA. Data (3 replicates per condition) are shown as means ± s.e.m. *, P ≤ 0.05 **, P ≤ 0.005 (calculated by one-way ANOVA with Bonferroni correction). Results are representative of 3 independent experiments.
Comment in
- IL-17-producing B cells combat parasites.
León B, Lund FE. León B, et al. Nat Immunol. 2013 May;14(5):419-21. doi: 10.1038/ni.2593. Nat Immunol. 2013. PMID: 23598388 No abstract available.
References
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL075453/HL/NHLBI NIH HHS/United States
- R56 AI084457/AI/NIAID NIH HHS/United States
- K12HD043376/HD/NICHD NIH HHS/United States
- R01 HD037091/HD/NICHD NIH HHS/United States
- R01 AI084457/AI/NIAID NIH HHS/United States
- 5T32AR007108/AR/NIAMS NIH HHS/United States
- R01 AI075589/AI/NIAID NIH HHS/United States
- R56 AI071163/AI/NIAID NIH HHS/United States
- R01 AI071163/AI/NIAID NIH HHS/United States
- AI071163/AI/NIAID NIH HHS/United States
- HD037091/HD/NICHD NIH HHS/United States
- HL075453/HL/NHLBI NIH HHS/United States
- K12 HD043376/HD/NICHD NIH HHS/United States
- AI084457/AI/NIAID NIH HHS/United States
- T32 AR007108/AR/NIAMS NIH HHS/United States
- AI 075589/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous