Fingolimod phosphate attenuates oligomeric amyloid β-induced neurotoxicity via increased brain-derived neurotrophic factor expression in neurons - PubMed (original) (raw)
Fingolimod phosphate attenuates oligomeric amyloid β-induced neurotoxicity via increased brain-derived neurotrophic factor expression in neurons
Yukiko Doi et al. PLoS One. 2013.
Abstract
The neurodegenerative processes that underlie Alzheimer's disease are mediated, in part, by soluble oligomeric amyloid β, a neurotoxic protein that inhibits hippocampal long-term potentiation, disrupts synaptic plasticity, and induces the production of reactive oxygen species. Here we show that the sphingosine-1-phosphate (S1P) receptor (S1PR) agonist fingolimod phosphate (FTY720-P)-a new oral drug for multiple sclerosis-protects neurons against oligomeric amyloid β-induced neurotoxicity. We confirmed that primary mouse cortical neurons express all of the S1P receptor subtypes and FTY720-P directly affects the neurons. Treatment with FTY720-P enhanced the expression of brain-derived neurotrophic factor (BDNF) in neurons. Moreover, blocking BDNF-TrkB signaling with a BDNF scavenger, TrkB inhibitor, or ERK1/2 inhibitor almost completely ablated these neuroprotective effects. These results suggested that the neuroprotective effects of FTY720-P are mediated by upregulated neuronal BDNF levels. Therefore, FTY720-P may be a promising therapeutic agent for neurodegenerative diseases, such as Alzheimer's disease.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
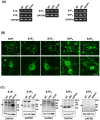
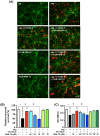
Figure 1. Neurons express all S1PR subtypes.
(A) Representative RT-PCR data for S1PRs. Neurons constitutively expressed all S1PR subtypes regardless of oAβ–stimulation. (B) Immunostaining data for S1PRs in untreated neurons. Neurons constitutively expressed all S1PR subtypes. Scale bar, 20 µm. (C) Western blotting data for S1PRs. Arrowheads indicate the bands for S1PRs. Neurons constitutively expressed all S1PR subtypes regardless of oAβ–stimulation. NT, no treatment; oA 5 µM oAβ1-42 treatment. The positive control samples used for each S1PR were as follows; brain for S1P1 and S1P3; heart for S1P2; spleen for S1P4 and S1P5.
5 µM oAβ1-42 treatment. The positive control samples used for each S1PR were as follows; brain for S1P1 and S1P3; heart for S1P2; spleen for S1P4 and S1P5.
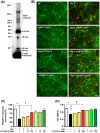
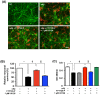
Figure 2. FTY720-P suppressed oAβ–induced neurotoxicity.
(A) Western blotting image for characterization of oAβ ˜ Data showed Aβ1-42 oligomerization composed with small (monomer, dimer, 3-mer, 4-mer, 8-mer, and 12-mer) and large oligomers. (B) Fluorescent microscopic images of mouse primary cortical neuron cultures. Treatment with FTY720-P was neuroprotective against oAβ–mediated toxicity. Neurons were stained with anti–MAP-2 (green) antibodies and Aβ was stained with 4G8 antibodies` (red). Scale bar: 50 µm. NT, no treatment; oA 5 µM oAβ1-42treatment. (C) Relative neuronal survival. The number of viable neurons (MAP-2–positive neurons) was quantified relative to results observed with untreated neurons. FTY720-P rescued neurons against oAβ–mediated toxicity. oA
5 µM oAβ1-42treatment. (C) Relative neuronal survival. The number of viable neurons (MAP-2–positive neurons) was quantified relative to results observed with untreated neurons. FTY720-P rescued neurons against oAβ–mediated toxicity. oA 5 µM oAβ1-42treatment. *, P<0.001; †, P<0.001. Values are means±SEM (n = 3). (D) WST-8 assay. FTY720-P enhanced neuronal survival against oAβ–mediated toxicity. oA
5 µM oAβ1-42treatment. *, P<0.001; †, P<0.001. Values are means±SEM (n = 3). (D) WST-8 assay. FTY720-P enhanced neuronal survival against oAβ–mediated toxicity. oA 5 µM oAβ1-42treatment. *, P<0.001; †, P<0.001. Values are means ± SEM (n = 6).
5 µM oAβ1-42treatment. *, P<0.001; †, P<0.001. Values are means ± SEM (n = 6).
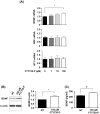
Figure 3. FTY720-P enhanced BDNF production.
(A) qRT-PCR data for neurotrophic factors in neurons. FTY720-P significantly upregulated mRNA expression levels of BDNF. *, P<0.05. Values are means ± SEM (n = 6). (B) Western blotting data for BDNF production. FTY720-P significantly enhanced neuronal BDNF production. BDNF levels are quantified relative to those in untreated neurons. NT, no treatment. *, P<0.05. Values are means ± SEM (n = 6). (C) ELISA data for BDNF production. BDNF levels significantly increased in response to FTY720-P. NT, no treatment. †, P<0.001. Values are means ± SEM (n = 3).
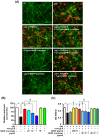
Figure 4. Neuroprotective effects of FTY720-P depended on BDNF.
(A) Fluorescent microscopic images of mouse primary cortical neuron cultures. The BDNF scavenger canceled the neuroprotective effects of FTY720-P. Neurons were stained with anti–MAP-2 antibodies (green) and Aβ was stained with 4G8 antibodies (red). Scale bar: 50 µm. NT, no treatment; oA 5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. (B) Relative neuronal survival. The number of viable neurons (MAP-2–positive neurons) was quantified relative to results obtained with untreated neurons. The BDNF scavenger dose-dependently suppressed the neuroprotective effects of FTY720-P. oA
5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. (B) Relative neuronal survival. The number of viable neurons (MAP-2–positive neurons) was quantified relative to results obtained with untreated neurons. The BDNF scavenger dose-dependently suppressed the neuroprotective effects of FTY720-P. oA 5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.01; §, P<0.001. Values are means ± SEM (n = 3). (C) WST-8 assay. Addition of BDNF enhanced neuronal survival against oAβ–mediated toxicity to the same extent of FTY720-P. The BDNF scavenger reversed the neuroprotective effects of FTY720-P in a dose-dependent manner, whereas it did not enhance oAβ–mediated neurotoxicity. oA
5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.01; §, P<0.001. Values are means ± SEM (n = 3). (C) WST-8 assay. Addition of BDNF enhanced neuronal survival against oAβ–mediated toxicity to the same extent of FTY720-P. The BDNF scavenger reversed the neuroprotective effects of FTY720-P in a dose-dependent manner, whereas it did not enhance oAβ–mediated neurotoxicity. oA 5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.01; §, P<0.001. Values are means ± SEM (n = 6).
5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.01; §, P<0.001. Values are means ± SEM (n = 6).
Figure 5. Activation of TrkB by BDNF is critical for the neuroprotective effects of FTY720-P.
(A) Fluorescent microscopic images of mouse primary cortical neuron cultures. The TrkB inhibitor ANA-12 ablated the neuroprotective effects of FTY720-P against oAβ-induced neurotoxicity. Neurons were stained with anti–MAP-2 antibodies (green) and Aβ was stained with 4G8 antibodies (red). Scale bar: 50 µm. NT, no treatment; oA 5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. (B) Relative neuronal survival. The number of viable neurons (MAP-2–positive neurons) was quantified relative to results observed in untreated neurons. TrkB inhibition almost completely inhibited the neuroprotective effects of FTY720-P. oA
5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. (B) Relative neuronal survival. The number of viable neurons (MAP-2–positive neurons) was quantified relative to results observed in untreated neurons. TrkB inhibition almost completely inhibited the neuroprotective effects of FTY720-P. oA 5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.001. Values are means ± SEM (n = 4). (C) WST-8 assay. Blocking TrkB almost completely reversed the neuroprotective effects of FTY720-P, whereas it did not enhance oAβ–mediated neurotoxicity. oA
5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.001. Values are means ± SEM (n = 4). (C) WST-8 assay. Blocking TrkB almost completely reversed the neuroprotective effects of FTY720-P, whereas it did not enhance oAβ–mediated neurotoxicity. oA 5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.001. Values are means ± SEM (n = 6).
5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.001. Values are means ± SEM (n = 6).
Figure 6. The neuroprotective effect of FTY720-P require ERK1/2 signaling.
(A) Fluorescent microscopic images of mouse primary cortical neuron cultures. ERK1/2 inhibitor U0126 almost completely suppressed FTY720-P–mediated protection against oAβ-induced neurotoxicity. Neurons were stained with anti–MAP-2 antibodies (green) and Aβ was stained with 4G8 antibodies (red). Scale bar: 50 µm. (B) Relative neuronal survival. The number of viable neurons (MAP-2–positive neurons) was quantified relative to results observed in untreated neurons. ERK1/2 inhibition almost completely ablated the neuroprotective effects of FTY720-P. oA 5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.001. Values are means ± SEM (n = 3). (C) WST-8 assay. Blocking ERK1/2 almost completely canceled the neuroprotective effects of FTY720-P, whereas it did not enhance oAβ–mediated neurotoxicity. oA
5 µM oAβ1-42treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.001. Values are means ± SEM (n = 3). (C) WST-8 assay. Blocking ERK1/2 almost completely canceled the neuroprotective effects of FTY720-P, whereas it did not enhance oAβ–mediated neurotoxicity. oA 5 µM oAβ1-42 treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.001. Values are means ± SEM (n = 6).
5 µM oAβ1-42 treatment; FTY720-P, 100 pM FTY720-P treatment. *, P<0.001; †, P<0.001; ‡, P<0.001. Values are means ± SEM (n = 6).
Similar articles
- Sphingosine 1-phosphate but not Fingolimod protects neurons against excitotoxic cell death by inducing neurotrophic gene expression in astrocytes.
Tran C, Heng B, Teo JD, Humphrey SJ, Qi Y, Couttas TA, Stefen H, Brettle M, Fath T, Guillemin GJ, Don AS. Tran C, et al. J Neurochem. 2020 Apr;153(2):173-188. doi: 10.1111/jnc.14917. Epub 2019 Dec 6. J Neurochem. 2020. PMID: 31742704 - Fingolimod increases brain-derived neurotrophic factor levels and ameliorates amyloid β-induced memory impairment.
Fukumoto K, Mizoguchi H, Takeuchi H, Horiuchi H, Kawanokuchi J, Jin S, Mizuno T, Suzumura A. Fukumoto K, et al. Behav Brain Res. 2014 Jul 15;268:88-93. doi: 10.1016/j.bbr.2014.03.046. Epub 2014 Apr 5. Behav Brain Res. 2014. PMID: 24713151 - Fingolimod protects cultured cortical neurons against excitotoxic death.
Di Menna L, Molinaro G, Di Nuzzo L, Riozzi B, Zappulla C, Pozzilli C, Turrini R, Caraci F, Copani A, Battaglia G, Nicoletti F, Bruno V. Di Menna L, et al. Pharmacol Res. 2013 Jan;67(1):1-9. doi: 10.1016/j.phrs.2012.10.004. Epub 2012 Oct 13. Pharmacol Res. 2013. PMID: 23073075 - Beneficial Effects of Fingolimod in Alzheimer's Disease: Molecular Mechanisms and Therapeutic Potential.
Angelopoulou E, Piperi C. Angelopoulou E, et al. Neuromolecular Med. 2019 Sep;21(3):227-238. doi: 10.1007/s12017-019-08558-2. Epub 2019 Jul 16. Neuromolecular Med. 2019. PMID: 31313064 Review. - Phosphorylation of the immunomodulator FTY720 inhibits programmed cell death of fibroblasts via the S1P3 receptor subtype and Bcl-2 activation.
Potteck H, Nieuwenhuis B, Lüth A, van der Giet M, Kleuser B. Potteck H, et al. Cell Physiol Biochem. 2010;26(1):67-78. doi: 10.1159/000315107. Epub 2010 May 18. Cell Physiol Biochem. 2010. PMID: 20502006 Review.
Cited by
- FTY720 attenuates excitotoxicity and neuroinflammation.
Cipriani R, Chara JC, Rodríguez-Antigüedad A, Matute C. Cipriani R, et al. J Neuroinflammation. 2015 May 8;12:86. doi: 10.1186/s12974-015-0308-6. J Neuroinflammation. 2015. PMID: 25953296 Free PMC article. - Phospholipid and Lipid Derivatives as Potential Neuroprotective Compounds.
Tayebati SK. Tayebati SK. Molecules. 2018 Sep 5;23(9):2257. doi: 10.3390/molecules23092257. Molecules. 2018. PMID: 30189584 Free PMC article. Review. - Loss of the neuroprotective factor Sphingosine 1-phosphate early in Alzheimer's disease pathogenesis.
Couttas TA, Kain N, Daniels B, Lim XY, Shepherd C, Kril J, Pickford R, Li H, Garner B, Don AS. Couttas TA, et al. Acta Neuropathol Commun. 2014 Jan 23;2:9. doi: 10.1186/2051-5960-2-9. Acta Neuropathol Commun. 2014. PMID: 24456642 Free PMC article. - Sphingosine-1-Phosphate Receptors Modulators Decrease Signs of Neuroinflammation and Prevent Parkinson's Disease Symptoms in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Mouse Model.
Pépin É, Jalinier T, Lemieux GL, Massicotte G, Cyr M. Pépin É, et al. Front Pharmacol. 2020 Feb 21;11:77. doi: 10.3389/fphar.2020.00077. eCollection 2020. Front Pharmacol. 2020. PMID: 32153401 Free PMC article. - The Direct Effects of Fingolimod in the Central Nervous System: Implications for Relapsing Multiple Sclerosis.
Hunter SF, Bowen JD, Reder AT. Hunter SF, et al. CNS Drugs. 2016 Feb;30(2):135-47. doi: 10.1007/s40263-015-0297-0. CNS Drugs. 2016. PMID: 26715391 Free PMC article. Review.
References
- Walsh DM, Teplow DB (2012) Alzheimer's disease and the amyloid beta-protein. Prog Mol Biol Transl Sci 107: 101–124. - PubMed
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, et al. (2007) Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem 282: 11590–11601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported in part by the Advanced Research for Medical Products Mining Program of the National Institute of Biomedical Innovation (NIBIO); a Grant-in-Aid for the global COE program from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and grants from the Ministry of Health, Labour and Welfare of Japan. he funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous