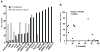Serine substitution of proline at codon 151 of TP53 confers gain of function activity leading to anoikis resistance and tumor progression of head and neck cancer cells - PubMed (original) (raw)
Comparative Study
. 2013 Jun;123(6):1416-23.
doi: 10.1002/lary.23846. Epub 2013 Apr 26.
Affiliations
- PMID: 23625637
- PMCID: PMC3664100
- DOI: 10.1002/lary.23846
Comparative Study
Serine substitution of proline at codon 151 of TP53 confers gain of function activity leading to anoikis resistance and tumor progression of head and neck cancer cells
Tong-Xin Xie et al. Laryngoscope. 2013 Jun.
Abstract
Objectives/hypothesis: Mutation of the TP53 gene occurs in more than half of cases of head and neck squamous cell carcinoma (HNSCC). However, little is known about how specific TP53 mutations affect tumor progression. The objective of this study is to determine the gain of function of mutant p53 with a proline-to-serine substitution at codon 151.
Study design: Laboratory-based study.
Methods: A panel of HNSCC cell lines was determined with anoikis assays, and orthotopic mouse experiments were performed. TP53 was sequenced. The shRNA knockdown and overexpression approaches were used for testing mutant p53 functions. The crystal structure of the p53 protein was analyzed using an in silico approach.
Results: An anoikis-resistant cell line, Tu138, was found to have a proline-to-serine substitution at codon 151 of TP53, which results in loss of wild-type p53 transcriptional activity. Moreover, the mutant p53 was shown to promote anoikis resistance and soft agar growth. Using an in silico approach based on the crystal structure of wild-type p53 protein, substitution of proline by serine at position 151 would create a cavity in a hydrophobic pocket, the loss of van der Waals contacts, and the thermodynamically unfavorable placement of a polar group, the hydroxyl oxygen atom of the serine, within a hydrophobic region, all of which likely cause a locally altered structure.
Conclusions: Our data suggest that mutation at position 151 leads to a structural alteration, which results in significant functional changes in the p53 protein that impact tumor progression.
Copyright © 2012 The American Laryngological, Rhinological and Otological Society, Inc.
Conflict of interest statement
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Figures
Figure 1
The top 8 anoikis-resistant and 8 anoikis sensitive cell lines were selected and injected into the tongues of nude mice. The association of anoikis resistance (detached for 48 hrs) with tumor size at 32 days after injection was analyzed.
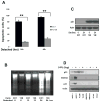
Figure 2
A. Tu138 and UMSCC1 cells were cultured in detached conditions for 24 and 48 hrs and dead cells were determined using Trypan-blue (** p<0.01, n=3). B. Tu138 and UMSCC1 cells were detached for 6, 12 and 24 hrs and fragmented DNA was isolated and subjected to agarose gel electrophoresis. C. The p53 expression in Tu138 and UMSCC1 cells was determined with Western blot after normal and detached culture for 24 hrs. D. Tu138, UMSCC1 and UMSCC1c2 cells were treated with 5ug/ml 5-FU for 20 hrs and the wild-type p53 MCF-7 cells were used as control. The p53 target genes p21 and MDM2 were examined using Western blotting.
Figure 3
p53P151S makes fewer inter residue contacts than wild type p53. (Left) Structure of wild type p53 in the vicinity of residue P151, Residue P151 and nearby interacting amino acid residues are shown as sticks and colored according to atom type whereby oxygen is red, nitrogen, blue and carbon, white. Dashed yellow lines indicate contacts between residue P151 and nearby residues (d ≤ 4.2 Å). (Right) The structure of the modeled p53P151S mutant, colored and demarked as in the left panel. The closest approach of the side chains of S151 and V147 is 5.8 Å.
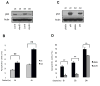
Figure 4
P53P151S can confer anoikis resistance. Western blot was performed to detect p53 knockdown effect in stable Tu138 cells after normal and detached culture for 24 hrs. B. Tu138 p53 knockdown cells and vector control cells were detached for 24 and 48 hrs and dead cells were determined with Trypan Blue (**, p<0.01, n=3). C. UMSCC1 cells were infected with retroviral vector pBaBe containing p53P151S cloned from Tu138 cells and empty vector infected cells as control. The stable cells were cultured in attached and detached conditions for 24 hrs. D. Anoikis assay was performed in UMSCC1 cells expressing p53P151S and their control cells (**, p<0.01, n=3).
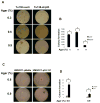
Figure 5
Mutant p53P151S promotes cell growth in soft agar. P53-knockdown Tu138 cells and UMSCC1 cells overexpressing mutant p53 and their control cells were cultured in soft agar for 14 days (for Tu138 cells, A and B. **, p<0.01, n=3) and 21 days (for UMSCC1cells, 5C and D. **, p<0.01, n=3).
Similar articles
- TP53 Mutations in Head and Neck Squamous Cell Carcinoma and Their Impact on Disease Progression and Treatment Response.
Zhou G, Liu Z, Myers JN. Zhou G, et al. J Cell Biochem. 2016 Dec;117(12):2682-2692. doi: 10.1002/jcb.25592. Epub 2016 Jun 3. J Cell Biochem. 2016. PMID: 27166782 Free PMC article. Review. - Disruptive TP53 mutation is associated with aggressive disease characteristics in an orthotopic murine model of oral tongue cancer.
Sano D, Xie TX, Ow TJ, Zhao M, Pickering CR, Zhou G, Sandulache VC, Wheeler DA, Gibbs RA, Caulin C, Myers JN. Sano D, et al. Clin Cancer Res. 2011 Nov 1;17(21):6658-70. doi: 10.1158/1078-0432.CCR-11-0046. Epub 2011 Sep 8. Clin Cancer Res. 2011. PMID: 21903770 Free PMC article. - p53-targeted lincRNA-p21 acts as a tumor suppressor by inhibiting JAK2/STAT3 signaling pathways in head and neck squamous cell carcinoma.
Jin S, Yang X, Li J, Yang W, Ma H, Zhang Z. Jin S, et al. Mol Cancer. 2019 Mar 11;18(1):38. doi: 10.1186/s12943-019-0993-3. Mol Cancer. 2019. PMID: 30857539 Free PMC article. - Chk1/2 inhibition overcomes the cisplatin resistance of head and neck cancer cells secondary to the loss of functional p53.
Gadhikar MA, Sciuto MR, Alves MV, Pickering CR, Osman AA, Neskey DM, Zhao M, Fitzgerald AL, Myers JN, Frederick MJ. Gadhikar MA, et al. Mol Cancer Ther. 2013 Sep;12(9):1860-73. doi: 10.1158/1535-7163.MCT-13-0157. Epub 2013 Jul 9. Mol Cancer Ther. 2013. PMID: 23839309 Free PMC article. - TP53 mutations in head and neck cancer.
Nathan CA, Khandelwal AR, Wolf GT, Rodrigo JP, Mäkitie AA, Saba NF, Forastiere AA, Bradford CR, Ferlito A. Nathan CA, et al. Mol Carcinog. 2022 Apr;61(4):385-391. doi: 10.1002/mc.23385. Epub 2022 Feb 23. Mol Carcinog. 2022. PMID: 35218075 Review.
Cited by
- Activation of Casein Kinase II by Gallic Acid Induces BIK-BAX/BAK-Mediated ER Ca++-ROS-Dependent Apoptosis of Human Oral Cancer Cells.
Lin ML, Chen SS. Lin ML, et al. Front Physiol. 2017 Sep 29;8:761. doi: 10.3389/fphys.2017.00761. eCollection 2017. Front Physiol. 2017. PMID: 29033852 Free PMC article. - Small-Molecule MMRi62 Induces Ferroptosis and Inhibits Metastasis in Pancreatic Cancer via Degradation of Ferritin Heavy Chain and Mutant p53.
Li J, Lama R, Galster SL, Inigo JR, Wu J, Chandra D, Chemler SR, Wang X. Li J, et al. Mol Cancer Ther. 2022 Apr 1;21(4):535-545. doi: 10.1158/1535-7163.MCT-21-0728. Mol Cancer Ther. 2022. PMID: 35131878 Free PMC article. - Oncomorphic _TP_53 Mutations in Gynecologic Cancers Lose the Normal Protein:Protein Interactions with the microRNA Microprocessing Complex.
Brachova P, Mueting SR, Devor EJ, Leslie KK. Brachova P, et al. J Cancer Ther. 2014 Jun 1;5(6):506-516. doi: 10.4236/jct.2014.56058. J Cancer Ther. 2014. PMID: 25339994 Free PMC article. - Docosahexaenoic acid inhibits the proliferation of Kras/TP53 double mutant pancreatic ductal adenocarcinoma cells through modulation of glutathione level and suppression of nucleotide synthesis.
Hung WC, Lee DY, Chiang EI, Syu JN, Chao CY, Yang MD, Tsai SY, Tang FY. Hung WC, et al. PLoS One. 2020 Nov 2;15(11):e0241186. doi: 10.1371/journal.pone.0241186. eCollection 2020. PLoS One. 2020. PMID: 33137095 Free PMC article. - Droplet digital polymerase chain reaction for detection and quantification of cell-free DNA TP53 target somatic mutations in oral cancer.
Lin LH, Cheng HW, Liu CJ. Lin LH, et al. Cancer Biomark. 2022;33(1):29-41. doi: 10.3233/CBM-210275. Cancer Biomark. 2022. PMID: 34366328 Free PMC article.
References
- Blons H, Laurent-Puig P. TP53 and head and neck neoplasms. Hum Mutat. 2003;21:252–257. - PubMed
- Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000;77:81–137. - PubMed
- Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28(6):622–629. Version of the database (R14, November 2009). http://www-p53.iarc.fr/ - PubMed
- Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002;19:607–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- P50 CA097007/CA/NCI NIH HHS/United States
- CA016672/CA/NCI NIH HHS/United States
- R01 DE014613/DE/NIDCR NIH HHS/United States
- P50CA097007/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous