Activating Mutations in ERBB2 and Their Impact on Diagnostics and Treatment - PubMed (original) (raw)
Activating Mutations in ERBB2 and Their Impact on Diagnostics and Treatment
Grit S Herter-Sprie et al. Front Oncol. 2013.
Abstract
Despite the ongoing "war on cancer," cancer remains one of the major causes of human morbidity and mortality. A new paradigm of targeted therapies holds the most promise for the future, making identification of tumor-specific therapeutic targets of prime importance. ERBB2/HER2, best known for its role in breast cancer tumorigenesis, can be targeted by two types of pharmacological manipulation: antibody therapy against the extracellular receptor domain and small molecule compounds against the intracellular tyrosine kinase domain. Aberrant activation of ERBB2 by gene amplification has been shown to participate in the pathophysiology of breast, ovarian, gastric, colorectal, lung, brain, and head and neck tumors. However, the advent of next-generation sequencing technologies has enabled efficient identification of activating molecular alterations of ERBB2. In this review, we will focus on the functional role of these somatic mutations that cause ERBB2 receptor activation. We will additionally discuss the current preclinical and clinical therapeutic strategies for targeting mutationally activated ERBB2.
Keywords: ERBB2/HER2; activating somatic mutation; breast cancer; lung cancer; resistance; reversible and irreversible tyrosine kinase inhibitors; targeted therapies.
Figures
Figure 1
Somatic activating mutations in ERBB2. Depicted is a simplified schematic of three known subclasses of ERBB2-mutants. (A) and (B) Activating mutations in the full-length protein. A star indicates the position of the activating mutation (A in the kinase domain, B in the extracellular domain). (C) Large deletions of the extracellular domain that yield the trucated form of ERBB2, p95HER2.
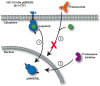
Figure 2
Mechanism of resistance upon continuous lapatinib treatment. Depicted is a simplified schematic of lapatinib-induced resistance toward current anti-ERBB2 therapeutics as identified by Xia et al. (2011). Continuous inhibition of 611-CTF with lapatinib induces nuclear p95HER2L expression (1). Trastuzumab and lapatinib are ineffective in targeting nuclear p95HER2L, thereby failing to control oncogenic proliferation (2). As formation of p95HER2L potentially depends on proteasomal processing, proteasome inhibition effectively prevents p95HER2L emergence (3).
References
- Anglesio M. S., Arnold J. M., George J., Tinker A. V., Tothill R., Waddell N., et al. (2008). Mutation of ERBB2 provides a novel alternative mechanism for the ubiquitous activation of RAS-MAPK in ovarian serous low malignant potential tumors. Mol. Cancer Res. 6, 1678–1690 10.1158/1541-7786.MCR-08-0193 - DOI - PMC - PubMed
- Arcila M. E., Chaft J. E., Nafa K., Roy-Chowdhuri S., Lau C., Zaidinski M., et al. (2012). Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin. Cancer Res. 18, 4910–4918 10.1158/1078-0432.CCR-12-0912 - DOI - PMC - PubMed
Grants and funding
- R01 CA122794/CA/NCI NIH HHS/United States
- R01 CA163896/CA/NCI NIH HHS/United States
- R01 CA166480/CA/NCI NIH HHS/United States
- R01 CA140594/CA/NCI NIH HHS/United States
- U01 CA141576/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous