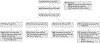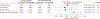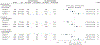Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4 - PubMed (original) (raw)
Randomized Controlled Trial
. 2013 Jul;131(7):843-50.
doi: 10.1001/jamaophthalmol.2013.4412.
Emily Y Chew, John Paul SanGiovanni, Frederick L Ferris, Wai T Wong, Elvira Agron, Traci E Clemons, Robert Sperduto, Ronald Danis, Suresh R Chandra, Barbara A Blodi, Amitha Domalpally, Michael J Elman, Andrew N Antoszyk, Alan J Ruby, David Orth, Susan B Bressler, Gary E Fish, George B Hubbard, Michael L Klein, Thomas R Friberg, Philip J Rosenfeld, Cynthia A Toth, Paul Bernstein
Collaborators, Affiliations
- PMID: 23645227
- PMCID: PMC6774801
- DOI: 10.1001/jamaophthalmol.2013.4412
Randomized Controlled Trial
Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4
Age-Related Eye Disease Study 2 (AREDS2) Research Group et al. JAMA Ophthalmol. 2013 Jul.
Abstract
Importance: Age-related cataract is a leading cause of visual impairment in the United States. The prevalence of age-related cataract is increasing, with an estimated 30.1 million Americans likely to be affected by 2020.
Objective: To determine whether daily oral supplementation with lutein/zeaxanthin affects the risk for cataract surgery.
Design, setting, and patients: The Age-Related Eye Disease Study 2 (AREDS2), a multicenter, double-masked clinical trial, enrolled 4203 participants, aged 50 to 85 years, at risk for progression to advanced age-related macular degeneration.
Interventions: Participants were randomly assigned to daily placebo; lutein/zeaxanthin, 10mg/2mg; omega-3 long-chain polyunsaturated fatty acids, 1 g; or a combination to evaluate the effects on the primary outcome of progression to advanced age-related macular degeneration.
Main outcomes and measures: Cataract surgery was documented at annual study examination with the presence of pseudophakia or aphakia, or reported during telephone calls at 6-month intervals between study visits. Annual best-corrected visual acuity testing was performed. A secondary outcome of AREDS2 was to evaluate the effects of lutein/zeaxanthin on the subsequent need for cataract surgery.
Results: A total of 3159 AREDS2 participants were phakic in at least 1 eye and 1389 of 6027 study eyes underwent cataract surgery during the study, with median follow-up of 4.7 years. The 5-year probability of progression to cataract surgery in the no lutein/zeaxanthin group was 24%. For lutein/zeaxanthin vs no lutein/zeaxanthin, the hazard ratios for progression to cataract surgery was 0.96 (95% CI, 0.84-1.10; P = .54). For participants in the lowest quintile of dietary intake of lutein/zeaxanthin, the hazard ratio comparing lutein/zeaxanthin vs no lutein/zeaxanthin for progression to cataract surgery was 0.68 (95% CI, 0.48-0.96; P = .03). The hazard ratio for 3 or more lines of vision loss was 1.03 (95% CI, 0.93-1.13; P = .61 for lutein/zeaxanthin vs no lutein/zeaxanthin).
Conclusions and relevance: Daily supplementation with lutein/zeaxanthin had no statistically significant overall effect on rates of cataract surgery or vision loss.
Trial registration: clinicaltrials.gov Identifier: NCT00345176.
Conflict of interest statement
Conflict of Interest Disclosures: None reported.
Figures
Figure 1.
Flow Diagram of Participants in the Age-Related Eye Disease Study 2 of Lutein/Zeaxanthin Treatment for Age-Related Cataract DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid.
Figure 2.
Progression to Cataract Surgery in Age-Related Eye Disease Study 2 Participants by Treatment (Lutein/Zeaxanthin vs No Lutein/Zeaxanthin)
Figure 3.
Effect of Lutein/Zeaxanthin Supplementation on the Progression of Cataract Stratified by Dietary Intake of Lutein/Zeaxanthin
Figure 4.
Effect of Lutein/Zeaxanthin Supplementation on Moderate Vision Loss (3 or More Lines) From Baseline Stratified by Dietary Intake of Lutein/Zeaxanthin IQR indicates interquartile range.
Similar articles
- Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3.
Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL 3rd, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agrón E, Toth CA, Bernstein PS, Sperduto RD. Age-Related Eye Disease Study 2 (AREDS2) Research Group, et al. JAMA Ophthalmol. 2014 Feb;132(2):142-9. doi: 10.1001/jamaophthalmol.2013.7376. JAMA Ophthalmol. 2014. PMID: 24310343 Free PMC article. Clinical Trial. - Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial.
Age-Related Eye Disease Study 2 Research Group. Age-Related Eye Disease Study 2 Research Group. JAMA. 2013 May 15;309(19):2005-15. doi: 10.1001/jama.2013.4997. JAMA. 2013. PMID: 23644932 Clinical Trial. - Association of Mortality with Ocular Diseases and Visual Impairment in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 Report Number 13.
Age-Related Eye Disease Study 2 Research Group; Papudesu C, Clemons TE, Agrón E, Chew EY. Age-Related Eye Disease Study 2 Research Group, et al. Ophthalmology. 2018 Apr;125(4):512-521. doi: 10.1016/j.ophtha.2017.10.028. Epub 2017 Nov 16. Ophthalmology. 2018. PMID: 29153456 Free PMC article. Clinical Trial. - Age-related Eye Disease Study 2: perspectives, recommendations, and unanswered questions.
Aronow ME, Chew EY. Aronow ME, et al. Curr Opin Ophthalmol. 2014 May;25(3):186-90. doi: 10.1097/ICU.0000000000000046. Curr Opin Ophthalmol. 2014. PMID: 24614146 Free PMC article. Review. - The Effect of Dietary Supplementations on Delaying the Progression of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis.
Csader S, Korhonen S, Kaarniranta K, Schwab U. Csader S, et al. Nutrients. 2022 Oct 13;14(20):4273. doi: 10.3390/nu14204273. Nutrients. 2022. PMID: 36296956 Free PMC article. Review.
Cited by
- Dietary Spirulina Supplementation Protects Visual Function From Photostress by Suppressing Retinal Neurodegeneration in Mice.
Okamoto T, Kawashima H, Osada H, Toda E, Homma K, Nagai N, Imai Y, Tsubota K, Ozawa Y. Okamoto T, et al. Transl Vis Sci Technol. 2019 Nov 20;8(6):20. doi: 10.1167/tvst.8.6.20. eCollection 2019 Nov. Transl Vis Sci Technol. 2019. PMID: 31788349 Free PMC article. - Aging and antioxidants: the impact of dietary carotenoid intakes on soluble klotho levels in aged adults.
He X, Yin X, Chen X, Chen X. He X, et al. Front Endocrinol (Lausanne). 2023 Oct 26;14:1283722. doi: 10.3389/fendo.2023.1283722. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37955004 Free PMC article. - The Association of Dietary Lutein plus Zeaxanthin and B Vitamins with Cataracts in the Age-Related Eye Disease Study: AREDS Report No. 37.
Glaser TS, Doss LE, Shih G, Nigam D, Sperduto RD, Ferris FL 3rd, Agrón E, Clemons TE, Chew EY; Age-Related Eye Disease Study Research Group. Glaser TS, et al. Ophthalmology. 2015 Jul;122(7):1471-9. doi: 10.1016/j.ophtha.2015.04.007. Epub 2015 May 9. Ophthalmology. 2015. PMID: 25972257 Free PMC article. Clinical Trial. - Nutrient Supplementation for Age-related Macular Degeneration, Cataract, and Dry Eye.
Hobbs RP, Bernstein PS. Hobbs RP, et al. J Ophthalmic Vis Res. 2014 Oct-Dec;9(4):487-93. doi: 10.4103/2008-322X.150829. J Ophthalmic Vis Res. 2014. PMID: 25709776 Free PMC article. Review. - Nutritional Supplements for Healthy Aging: A Critical Analysis Review.
Kaufman MW, DeParis S, Oppezzo M, Mah C, Roche M, Frehlich L, Fredericson M. Kaufman MW, et al. Am J Lifestyle Med. 2024 Apr 9:15598276241244725. doi: 10.1177/15598276241244725. Online ahead of print. Am J Lifestyle Med. 2024. PMID: 39554957 Free PMC article. Review.
References
- Pascolini D, Mariotti SP, Pokharel GP, et al. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004;11(2):67–115. - PubMed
- Congdon N, O’Colmain B, Klaver CC, et al.; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. - PubMed
- Congdon N, Vingerling JR, Klein BE, et al.; Eye Diseases Prevalence Research Group. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487–494. - PubMed
- Leske MC, Chylack LT Jr, Wu SY. The Lens Opacities Case-Control Study: risk factors for cataract. Arch Ophthalmol. 1991;109(2):244–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ZIA AT000018-02/ImNIH/Intramural NIH HHS/United States
- R01 EY021532/EY/NEI NIH HHS/United States
- ZIA EY000485-07/ImNIH/Intramural NIH HHS/United States
- N01-EY-5-0007/EY/NEI NIH HHS/United States
- HHS-N-260-2005-00007-C/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical