Dysmyelination of auditory afferent axons increases the jitter of action potential timing during high-frequency firing - PubMed (original) (raw)
Dysmyelination of auditory afferent axons increases the jitter of action potential timing during high-frequency firing
Jun Hee Kim et al. J Neurosci. 2013.
Abstract
Auditory neuropathies are linked to loss of temporal acuity of sound-evoked signals, which may be related to myelin loss. However, it is not known how myelin loss affects the waveform and temporal precision of action potentials (APs) in auditory CNS nerve terminals. Here we investigated the excitability of the calyx of Held nerve terminal in dysmyelinated auditory brainstems using the Long-Evans Shaker (LES) rat, a spontaneous mutant where compact myelin wrapping does not occur due to a genetic deletion of myelin basic protein. We found at relatively mature postnatal ages (15-17 d after birth) LES rat calyces showed prolonged spike latencies, indicative of a threefold reduction in the AP propagation velocity. Furthermore, LES rat afferent fiber-evoked APs showed a pronounced loss of temporal precision, even at low stimulation frequencies (10 Hz). While normal calyces were able to fire APs without failures at impressive rates of up to 1 kHz, LES calyces were unable to do so. Direct recordings of the presynaptic calyx terminal AP waveform revealed that myelin loss does not affect the AP spike upstroke and downstroke kinetics, but dysmyelination reduces the after-depolarization and enhances the fast after-hyperpolarization peak following the AP spike in the LES rat. Together these findings show that proper myelination is essential not only for fast AP propagation, but also for precise presynaptic AP firing that minimizes both spike jitter and failures, two characteristics critically important for the accurate processing of sound signals in the auditory brainstem.
Figures
Figure 1.
Expression of MBP in the rodent auditory brainstem increases with age. Fixed slices of auditory brainstem containing the ventral stria and MNTB were stained for myelin (MBP; green) and for synaptic vesicles (VGluT1; red). A, In normal young rats (LE rat; P5), sparse myelin is present in axonal projections. B, Soon after the onset of hearing (P13), myelin is highly expressed in MNTB axons, and terminates close to the calyx of Held presynaptic terminal. The arrow indicates the midline, where the bipolar stimulus electrode was placed. The areas containing the MNTB are indicated by dotted lines. C, Enlarged image of B, showing staining of presynaptic glutamatergic terminals and myelinated axons in the MNTB. D, Enlarged image of C, showing staining of presynaptic calyx-like terminals (yellow arrowheads) and myelinated axons in the MNTB. The white arrow shows a single isolated myelinated axon. E, A P17 mutant LES rat completely lacks staining for MBP, but synaptic vesicle staining (VGluT1; red) in the MNTB appears to be normal.
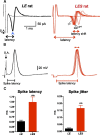
Figure 2.
LES rats show increased presynaptic spike latency and a lack of temporal precision during 10 Hz repetitive firing. A, Afferent fiber stimulation at 10 Hz (10 stimulus pulses) results in presynaptic extracellular spikes when recorded in the cell-attached mode at a P17 calyx terminal: normal LE (black trace) and the LES mutant rat (red trace). Note the large latency shift (and temporal jitter) in the LES rat spikes (the labels first, second, and last indicate the order of responses to 10 Hz afferent fiber stimulation). One subthreshold trace is shown. B, Presynaptic action potentials from the same calyx recordings as shown in A in whole-cell current-clamp mode. Note the lack of precision (or jitter) in the timing of the spikes for LES mutants. C, Summary of the spike delay and jitter in LE and LES rats (**p < 0.001). S.A., stimulus artifact.
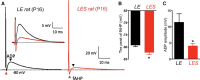
Figure 3.
LES calyx terminals have a smaller ADP and an enhanced fAHP peak. Afferent fiber stimulation produced an action potential with an ADP in a P16 normal LE calyx recording (black; A) and an ADP with a reduced peak value in a calyx from the LES (right, red trace). Both calyces displayed an ADP (black arrowhead) and fAHP (red arrowhead). Inset, Same traces on an expanded voltage scale. B, Peak reversal voltage of fAHP was significantly larger in calyces from LES than LE, recorded from P15–P18 rats. C, Summary of the ADP amplitude, measured from resting potential to the peak of ADP in normal LE and mutant LES calyces in P15–P18 rats.
Figure 4.
LES calyx terminals cannot follow APs at higher frequencies. AP trains were triggered at 100 Hz (A), 300 Hz (B), and 1 kHz (C) at room temperature (24°C, A and B) and at physiological temperature (PT; 37°C, C). The calyx of Held of both LE and LES rats can follow 100 Hz stimulation, but at higher stimulation frequencies (300 Hz and 1 kHz) presynaptic APs of the LES calyx terminals experience intermittent failures, whereas normal LE calyx terminals can routinely follow 300 Hz (at room temperature) and even 1 kHz at 37°C without AP failures. Recordings were from P16 LE and LES rats (black and red traces, respectively). The horizontal dashed line indicates the resting membrane potential of −80 mV. Arrowheads in B and blue arrows in C indicate AP failures. LES rats showed more AP failures and the timing of the APs showed more “jitter.”
Similar articles
- Functional and structural properties of ion channels at the nerve terminal depends on compact myelin.
Berret E, Kim SE, Lee SY, Kushmerick C, Kim JH. Berret E, et al. J Physiol. 2016 Oct 1;594(19):5593-609. doi: 10.1113/JP272205. Epub 2016 Jul 18. J Physiol. 2016. PMID: 27168396 Free PMC article. - Central dysmyelination reduces the temporal fidelity of synaptic transmission and the reliability of postsynaptic firing during high-frequency stimulation.
Kim SE, Turkington K, Kushmerick C, Kim JH. Kim SE, et al. J Neurophysiol. 2013 Oct;110(7):1621-30. doi: 10.1152/jn.00117.2013. Epub 2013 Jul 10. J Neurophysiol. 2013. PMID: 23843435 Free PMC article. - Sound-Evoked Activity Influences Myelination of Brainstem Axons in the Trapezoid Body.
Sinclair JL, Fischl MJ, Alexandrova O, Heβ M, Grothe B, Leibold C, Kopp-Scheinpflug C. Sinclair JL, et al. J Neurosci. 2017 Aug 23;37(34):8239-8255. doi: 10.1523/JNEUROSCI.3728-16.2017. Epub 2017 Jul 31. J Neurosci. 2017. PMID: 28760859 Free PMC article. - Axon-oligodendrocyte interactions during developmental myelination, demyelination and repair.
Piaton G, Gould RM, Lubetzki C. Piaton G, et al. J Neurochem. 2010 Sep 1;114(5):1243-60. doi: 10.1111/j.1471-4159.2010.06831.x. Epub 2010 May 26. J Neurochem. 2010. PMID: 20524961 Review. - The calyx of Held in the auditory system: Structure, function, and development.
Baydyuk M, Xu J, Wu LG. Baydyuk M, et al. Hear Res. 2016 Aug;338:22-31. doi: 10.1016/j.heares.2016.03.009. Epub 2016 Mar 25. Hear Res. 2016. PMID: 27018297 Free PMC article. Review.
Cited by
- Persistent and resurgent Na+ currents in vestibular calyx afferents.
Meredith FL, Rennie KJ. Meredith FL, et al. J Neurophysiol. 2020 Aug 1;124(2):510-524. doi: 10.1152/jn.00124.2020. Epub 2020 Jul 15. J Neurophysiol. 2020. PMID: 32667253 Free PMC article. - Myelination Deficits in the Auditory Brainstem of a Mouse Model of Fragile X Syndrome.
Lucas A, Poleg S, Klug A, McCullagh EA. Lucas A, et al. Front Neurosci. 2021 Nov 11;15:772943. doi: 10.3389/fnins.2021.772943. eCollection 2021. Front Neurosci. 2021. PMID: 34858133 Free PMC article. - Myelination of Purkinje axons is critical for resilient synaptic transmission in the deep cerebellar nucleus.
Barron T, Saifetiarova J, Bhat MA, Kim JH. Barron T, et al. Sci Rep. 2018 Jan 18;8(1):1022. doi: 10.1038/s41598-018-19314-0. Sci Rep. 2018. PMID: 29348594 Free PMC article. - How Do Cells of the Oligodendrocyte Lineage Affect Neuronal Circuits to Influence Motor Function, Memory and Mood?
Pepper RE, Pitman KA, Cullen CL, Young KM. Pepper RE, et al. Front Cell Neurosci. 2018 Nov 16;12:399. doi: 10.3389/fncel.2018.00399. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30524235 Free PMC article. Review. - Revisiting the Chicken Auditory Brainstem Response: Frequency Specificity, Threshold Sensitivity, and Cross Species Comparison.
Ordiway G, McDonnell M, Sanchez JT. Ordiway G, et al. Neurosci Insights. 2024 Jan 30;19:26331055241228308. doi: 10.1177/26331055241228308. eCollection 2024. Neurosci Insights. 2024. PMID: 38304551 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous