Current Challenges for HER2 Testing in Diagnostic Pathology: State of the Art and Controversial Issues - PubMed (original) (raw)
Current Challenges for HER2 Testing in Diagnostic Pathology: State of the Art and Controversial Issues
Anna Sapino et al. Front Oncol. 2013.
Abstract
HER2 overexpression and anti-HER2 agents represent probably the best story of success of individualized therapy in breast cancer. Due to the important therapeutic implications, the issue under the spotlight has been, since ever, the correct identification of true HER2 positivity on tissue specimens. Eligibility to anti-HER2 agents is strictly dependent on the demonstration of HER2 overexpression (by immunohistochemistry) or of HER2 gene amplification by in situ techniques (fluorescence in situ hybridization, FISH), however there are controversial issues involving cases with "equivocal" HER2 status based on conventional techniques (about 20% of specimens). In terms of HER2 expression a major debate is the presence of full-length and truncated forms of the protein and controversial clinical data have been reported on the therapeutic implications of these HER2 fragments. In terms of HER2 gene assessment, the occurrence of amplification of the chromosome 17 centromeric region (CEP17) has been proven responsible for misleading HER2 FISH results, precluding anti-HER2 based therapy to some patients. Finally HER2 activating mutations have been recently described as a biological mechanisms alternative to HER2 gene amplification. In this review we will focus on the controversies that pathologists and oncologists routinely face in the attempt to design the most tailored treatment for breast cancer patients. We will focus on the HER2 gene and on the protein, both at technical and interpretational levels.
Keywords: CEP17 amplification; HER2; HER2 mutations; diagnosis; test; therapy; truncated HER2.
Figures
Figure 1
Steric hindrance phenomenon. Immunohistochemistry for the trastuzumab binding site (BiotHER staining) shows higher intensity in cell block sections of BT474 cells treated with 0.1% pronase and in pronase-treated (as “antigen retrieval”) cell block sections of BT474 cells. This is likely to be due to a matter of steric hindrance, according to which if antigen molecules are closely “packed” on the cell surface, spatial interference results in a greater likelihood of reduced antibody binding.
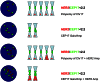
Figure 2
Chromosome 17 polysomy versus CEP17 gain/amplification. Possible scenarios in which additional CEP17 signals are encountered, from top to bottom: true chromosome 17 (chr17) polysomy without HER2 gene amplification; gain/amplification of CEP17 without HER2 amplification; HER2 amplification in a context of chr17 polysomy; HER2 amplification coupled with CEP17 gain/amplification. In all scenarios the ratio is below 2.2, however in the latter two cases HER2 amplification is present (HER2 mean copy number >6).
Similar articles
- Impact of the 2018 American Society of Clinical Oncology/College of American Pathologists HER2 Guideline Updates on HER2 Assessment in Breast Cancer With Equivocal HER2 Immunohistochemistry Results With Focus on Cases With HER2/CEP17 Ratio <2.0 and Average HER2 Copy Number ≥4.0 and <6.0.
Hoda RS, Brogi E, Xu J, Ventura K, Ross DS, Dang C, Robson M, Norton L, Morrow M, Wen HY. Hoda RS, et al. Arch Pathol Lab Med. 2020 May;144(5):597-601. doi: 10.5858/arpa.2019-0307-OA. Epub 2019 Oct 24. Arch Pathol Lab Med. 2020. PMID: 31647316 - Frequency of chromosome 17 polysomy in relation to CEP17 copy number in a large breast cancer cohort.
Koudelakova V, Trojanec R, Vrbkova J, Donevska S, Bouchalova K, Kolar Z, Varanasi L, Hajduch M. Koudelakova V, et al. Genes Chromosomes Cancer. 2016 May;55(5):409-17. doi: 10.1002/gcc.22337. Epub 2016 Feb 5. Genes Chromosomes Cancer. 2016. PMID: 26847577 - Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy.
Tse CH, Hwang HC, Goldstein LC, Kandalaft PL, Wiley JC, Kussick SJ, Gown AM. Tse CH, et al. J Clin Oncol. 2011 Nov 1;29(31):4168-74. doi: 10.1200/JCO.2011.36.0107. Epub 2011 Sep 26. J Clin Oncol. 2011. PMID: 21947821 - HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity.
Hanna WM, Rüschoff J, Bilous M, Coudry RA, Dowsett M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. Hanna WM, et al. Mod Pathol. 2014 Jan;27(1):4-18. doi: 10.1038/modpathol.2013.103. Epub 2013 Jun 28. Mod Pathol. 2014. PMID: 23807776 Review. - HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation.
Ahn S, Woo JW, Lee K, Park SY. Ahn S, et al. J Pathol Transl Med. 2020 Jan;54(1):34-44. doi: 10.4132/jptm.2019.11.03. Epub 2019 Nov 6. J Pathol Transl Med. 2020. PMID: 31693827 Free PMC article. Review.
Cited by
- TumorNext: A comprehensive tumor profiling assay that incorporates high resolution copy number analysis and germline status to improve testing accuracy.
Gray PN, Vuong H, Tsai P, Lu HM, Mu W, Hsuan V, Hoo J, Shah S, Uyeda L, Fox S, Patel H, Janicek M, Brown S, Dobrea L, Wagman L, Plimack E, Mehra R, Golemis EA, Bilusic M, Zibelman M, Elliott A. Gray PN, et al. Oncotarget. 2016 Oct 18;7(42):68206-68228. doi: 10.18632/oncotarget.11910. Oncotarget. 2016. PMID: 27626691 Free PMC article. - Is STARD3 A New Biomarker for Breast Cancer?
Korucu AN, Inandiklioglu N. Korucu AN, et al. Eur J Breast Health. 2024 Apr 1;20(2):89-93. doi: 10.4274/ejbh.galenos.2024.2024-1-7. eCollection 2024 Apr. Eur J Breast Health. 2024. PMID: 38571685 Free PMC article. Review. - Bioinformatics combined with clinical data to analyze clinical characteristics and prognosis in patients with HER2 low expression breast cancer.
Chen Y, Ma Y, Li Y, Yu Y, Lu B, Liao L, Li F, Wen Z, Jiang W, Guo P, Fang D, Lu G. Chen Y, et al. Gland Surg. 2023 Feb 28;12(2):197-207. doi: 10.21037/gs-22-747. Epub 2023 Feb 8. Gland Surg. 2023. PMID: 36915815 Free PMC article. - Multiplex Immunofluorescence and Multispectral Imaging: Forming the Basis of a Clinical Test Platform for Immuno-Oncology.
Hoyt CC. Hoyt CC. Front Mol Biosci. 2021 Jun 2;8:674747. doi: 10.3389/fmolb.2021.674747. eCollection 2021. Front Mol Biosci. 2021. PMID: 34150850 Free PMC article. Review. - High HER2 Intratumoral Heterogeneity Is a Predictive Factor for Poor Prognosis in Early-Stage and Locally Advanced HER2-Positive Breast Cancer.
Tanei T, Seno S, Sota Y, Hatano T, Kitahara Y, Abe K, Masunaga N, Tsukabe M, Yoshinami T, Miyake T, Shimoda M, Matsuda H, Shimazu K. Tanei T, et al. Cancers (Basel). 2024 Mar 5;16(5):1062. doi: 10.3390/cancers16051062. Cancers (Basel). 2024. PMID: 38473420 Free PMC article.
References
- Birner P., Oberhuber G., Stani J., Reithofer C., Samonigg H., Hausmaninger H. (2001). Evaluation of the United States Food and Drug Administration-approved scoring and test system of HER-2 protein expression in breast cancer. Clin. Cancer Res. 7, 1669–1675 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous