A Wee1 checkpoint inhibits anaphase onset - PubMed (original) (raw)
A Wee1 checkpoint inhibits anaphase onset
Noel Lianga et al. J Cell Biol. 2013.
Abstract
Cdk1 drives both mitotic entry and the metaphase-to-anaphase transition. Past work has shown that Wee1 inhibition of Cdk1 blocks mitotic entry. Here we show that the budding yeast Wee1 kinase, Swe1, also restrains the metaphase-to-anaphase transition by preventing Cdk1 phosphorylation and activation of the mitotic form of the anaphase-promoting complex/cyclosome (APC(Cdc20)). Deletion of SWE1 or its opposing phosphatase MIH1 (the budding yeast cdc25(+)) altered the timing of anaphase onset, and activation of the Swe1-dependent morphogenesis checkpoint or overexpression of Swe1 blocked cells in metaphase with reduced APC activity in vivo and in vitro. The morphogenesis checkpoint also depended on Cdc55, a regulatory subunit of protein phosphatase 2A (PP2A). cdc55Δ checkpoint defects were rescued by mutating 12 Cdk1 phosphorylation sites on the APC, demonstrating that the APC is a target of this checkpoint. These data suggest a model in which stepwise activation of Cdk1 and inhibition of PP2A(Cdc55) triggers anaphase onset.
Figures
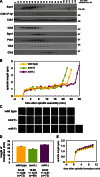
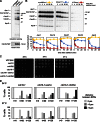
Figure 1.
Swe1 and Mih1 regulate anaphase onset. (A) Swe1 is present and active during mitosis. Wild-type (ADR4009) cells were arrested in G1 with 100 ng/ml α-factor, and released into the cell cycle (t = 0). 800 ng/ml α-factor was re-added at t = 55 min to arrest cells in the following G1. Samples for immunoblotting were taken at the indicated time points. (B–D) Swe1 inhibits and Mih1 promotes anaphase onset. Wild-type (ADR4009), _swe1_Δ (ADR4015), and _mih1_Δ (ADR4012) cells were arrested in G1 with 25 ng/ml α-factor, washed, and resuspended in synthetic media. After 25 min cells were placed on synthetic media agar pads and imaged using fluorescence microscopy. All strains were imaged on the same day and imaged on three separate occasions. Spindle length was determined by measuring the distance between spindle pole bodies tagged with Spc42-eGFP. Representative spindle measurements for cells progressing through mitosis are shown in B. Spc42-eGFP images for the spindles graphed in B are shown in C. (D) The length of metaphase (average ± SEM) was calculated for wild-type, _swe1_Δ, and _mih1_Δ cells by measuring the time spent between spindle formation and anaphase onset. Each pairwise comparison is significantly different (ANOVA, P < 0.0001, Tukey’s HSD = 2.1878 for P < 0.05). (E) Swe1 and Mih1 do not regulate spindle formation. The spindle lengths (average ± SEM) every minute after spindle formation were calculated from the wild-type, _swe1_Δ, and _mih1_Δ time courses in B.
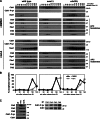
Figure 2.
The morphogenesis checkpoint regulates anaphase onset. (A and B) Morphogenesis checkpoint activation delays anaphase onset. Wild-type (ADR4009), _swe1_Δ (ADR4015), and cdk1-Y19F (ADR4313) cells were arrested in G1 with 25 ng/ml α-factor, washed, and resuspended in synthetic media. After 25 min cells were plated on synthetic media agar pads containing 2.5 µM latA or DMSO and imaged as in Fig. 1 B. (A) Spindle measurements for single representative wild-type and _swe1_Δ cells progressing through mitosis. (B) The length of time spent from spindle formation to anaphase onset was calculated for wild-type, _swe1_Δ, and cdk1-Y19F cells. Cells that spent >60 min with short spindles are characterized as “metaphase arrested,” whereas cells that spent <60 min with short spindles are characterized as “unarrested.” Wild-type and _swe1_Δ ± latA were imaged on three separate occasions and cdk1-Y19F ± latA were imaged on two separate occasions. The graph combines all data from all experiments. (C) Morphogenesis checkpoint activation does not perturb mitotic spindle formation. The spindle length (average ± SEM) every minute after spindle formation was calculated for all wild-type, _swe1_Δ, and cdk1-Y19F cells from B. (D) Morphogenesis checkpoint activation does not prevent spindle bipolarity. NDC80-eGFP (ADR5026) cells were grown and imaged as in A. The distance between separated Ndc80-eGFP foci was measured in cells entering anaphase (DMSO) and 150 min after α-factor wash-out (latA, inset). Gray lines are individual cell measurements; yellow and brown lines are average (±SEM) measurements.
Figure 3.
The morphogenesis checkpoint stabilizes APC substrates. (A and B) Activation of the morphogenesis checkpoint stabilizes APC substrates. Wild-type (ADR4009), _swe1_Δ (ADR4015), and _cdc55_Δ (ADR4738) cells were arrested in G1 with 100 ng/ml α-factor, washed, and released from the arrest (t = 0). Cells were treated with 2.5 µM latA or DMSO at t = 25 and 800 ng/ml α-factor was re-added at t = 65 to arrest cells in the following G1. Samples for immunoblotting (A) and fluorescence microscopy (B) were taken at the indicated time points. Anaphase onset was determined by scoring the distance between Spc42-eGFP–labeled SPBs in fixed cells. At least 200 cells were scored for each time point. (C) LatA-activated Swe1 does not preferentially phosphorylate a particular Cdk1–cyclin complex. Wild-type (ADR4006) cells were arrested with 100 ng/ml α-factor, 10 µg/ml nocodazole, or 2.5 µM latA. Cells were harvested for immunoblotting (left), or, for the latA-arrested cells, immunoprecipitation. Cdk1–Clb2, –Clb3 or –Clb5 complexes were immunoprecipitated, normalized for Cdk1 levels, and blotted for Cdk1 and Cdk1–P-tyr to compare the relative stoichiometry of tyrosine-phosphorylated Cdk1. Two independent experiments are shown, one that compares Clb3 and Clb5 immunoprecipitates (left) and one that compares Clb2 and Clb5 immunoprecipitates.
Figure 4.
Overexpression of Swe1 blocks cells in metaphase. (A) Swe1 overexpression and latA induce similar levels of tyrosine phosphorylation on Cdk1. Wild-type (ADR4009) cells were treated with latA and GAL-SWE1 (ADR4289) cells were grown in YEP + 2% raffinose (Raff) and induced by addition of 2% galactose (Gal). Cells were harvested and immunoblotted for Swe1, Cdk1, and Cdk1-P-tyr. (B and C) Overexpression of Swe1 in mitosis stabilizes APC substrates and arrests cells with 2N DNA content. Wild-type (ADR2617), GAL-SWE1 (ADR3871), and GAL-SWE1 cdk1-Y19F (ADR4228) cells were grown in YEP + 2% raffinose, arrested with 10 µg/ml nocodazole, and induced with 2% galactose (t = 0) for 1 h. Nocodazole was washed out, and cells released into YEP + 2% galactose. 25 ng/ml α-factor was added (t = 150) to arrest cells in the following G1. Samples were taken for immunoblotting (A) and flow cytometry (B) at the indicated time points. (D) Swe1 inhibits Clb-associated kinase activity. Cells were grown as in A, and Cdk1–Clb2, –Clb3 or –Clb5 complexes were immunoprecipitated with anti-Clb antibodies and their histone H1 kinase activity was measured to assess Cdk1 inhibition. (E) Overexpression of Swe1 impairs sister chromatid separation. Wild-type (ADR1393) and GAL-SWE1 (ADR1395) strains were grown as in A, except 1 mM CuSO4 was added at t = −30 to induce expression of GFP-lacI, and the separation of sister lacO arrays at TRP1 (∼12 kb from CENIV) was visualized by fluorescence microscopy. The graphs in D and E are representative of one of three repeats. (F) Overexpressed Swe1 does not preferentially phosphorylate a particular Cdk1–cyclin complex. GAL-SWE1 cells (ADR3871) were grown as in A, and Cdk1–Clb2, –Clb3, or –Clb5 complexes were immunoprecipitated at the indicated time points and analyzed by immunoblotting with anti-Cdk1 and anti–Cdk1-P-tyr antibodies. (G) Overexpression of Swe1 in anaphase restabilizes APCCdc20 substrates. cdc15-2 (ADR4252) and cdc15-2 GAL-SWE1 (ADR4245) cells were grown in YEP + 2% raffinose and arrested in anaphase by temperature shift to 35°C, followed by induction with 2% galactose (t = 0). Parallel cultures were arrested in 10 µg/ml nocodazole (noc) at 25°C to illustrate peak levels of APC substrates. Cdk1 was used as a loading control for three independent membranes loaded with identical samples.
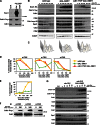
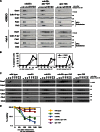
Figure 5.
Overexpression of Swe1 in mitosis inhibits APC phosphorylation in vivo and APCCdc20 activity in vitro. (A) Wild-type (ADR22), CDC16-TAP (ADR3877), and GAL-SWE1 CDC16-TAP (ADR3858) cells were grown in YEP + 2% raffinose, arrested in mitosis with 10 µg/ml nocodazole, and induced with 2% galactose for 90 min. Cells were then washed in medium lacking phosphate, and grown for 45 min in the presence of [32P]orthophosphate. The APC was purified, run on a polyacrylamide gel, and exposed to a phosphorimager screen or immunoblotted. (B) Overexpression of Swe1 inhibits ubiquitination of Pds1 in vitro. swe1Δ CDC16-TAP (ADR3877) and GAL-SWE1 CDC16-TAP (ADR3859) cells were grown in YEP + 2% raffinose, arrested in mitosis with 30 µg/ml benomyl, and induced with 2% galactose. The APC was purified and its activity assayed in an in vitro ubiquitination assay. Reactions were run on a polyacrylamide gel and exposed to a phosphorimager screen. The average activity (±SEM) of three independent experiments is plotted below a representative assay. APCCdc20 activity was quantified from assays containing methylated ubiquitin (Ub) and APCCdh1 activity was quantified from assays containing wild-type ubiquitin. (C) Cdk1–Clb2 kinase treatment reactivates Swe1-inhibited APC. APC was purified from swe1Δ CDC16-TAP and GAL-SWE1 CDC16-TAP cells as described in B. The APC was incubated with purified Cdk1–Clb2 complexes ± ATP before cleavage from IgG-coupled magnetic beads. The activity of the purified APC was then measured as in B. Quantification of a representative assay is shown below. Parallel samples were treated with γ-[32P]ATP to confirm efficient phosphorylation (
Fig. S5 H
). (D) CDC16-TAP (ADR3089), cdc16-6A-TAP (ADR3822), and cdc16-6A-TAP cdc27-5A (ADR3891) cells were arrested in mitosis with 30 µg/ml benomyl. Purified APC (
Fig. S5 I
) was phosphorylated by purified Cdk1–Clb2 complexes as in C and its activity measured. Quantification of a representative assay is shown below. Parallel samples were treated with γ-[32P]ATP to confirm efficient phosphorylation (
Fig. S5 J
). The experiments shown in B, C, and D are representative of one of two repeats. (E) mih1Δ lowers the maximum permissive temperature of _cdc20_-3. Eightfold serial dilutions of wild-type (ADR22), mih1Δ (ADR3178), swe1Δ (ADR3170), cdc20-3 (ADR3161), mih1Δ cdc20-3 (ADR3149), swe1Δ cdc20-3 (ADR3155), and swe1Δ mih1Δ cdc20-3 (ADR3141) cells were spotted onto YEPD plates and grown at the indicated temperatures.
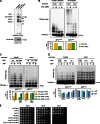
Figure 6.
PP2ACdc55 regulates APC phosphorylation and activity in vivo. (A) The APC is hyper-phosphorylated in vivo in cdc55Δ cells. cdc15-2 (ADR1168), cdc15-2 CDC16-TAP (ADR5137), and cdc15-2 cdc55Δ CDC16-TAP (ADR4060) cells were arrested in G1 with 100 ng/ml α-factor, washed, and released at 37°C. After 2.5 h cells were washed in medium lacking phosphate, and grown for 45 min in the presence of [32P]orthophosphate at 37°C. The APC was purified, run on a polyacrylamide gel, and exposed to a phosphorimager screen or immunoblotted. (B) PP2ACdc55 dephosphorylates the APC in vitro. The APC was purified from CDC16-TAP (ADR3089) cells and phosphorylated with purified Cdk1/Clb2 and γ-[32P]ATP while immobilized on IgG-coupled magnetic beads. The beads were washed and then incubated for the indicated times at room temperature with no addition (yellow lines), TAP-purified PP2ACdc55 (blue lines), or lambda phosphatase (red lines). The three reactions share a t = 0 sample that was taken before additions. The dephosphorylation of individual APC subunits was quantified and graphed relative to t = 0. The experiment shown is representative of one of three repeats. (C) cdc55Δ, but not swe1Δ, suppresses the temperature sensitivity of an APC mutant. Eightfold serial dilutions of wild-type (ADR4009), swe1Δ (ADR4015), cdc55Δ (ADR4738), cdc16-1 (ADR4979), cdc16-1 swe1Δ (ADR4984), and cdc16-1 cdc55Δ (ADR5020) cells were spotted onto plates and grown at the indicated temperatures. (D) cdc55Δ, but not swe1Δ, allows premature anaphase spindle elongation in cells with an impaired APC, but does not bypass APC function. cdc16-1 (ADR4979), _cdc16-1 swe1_Δ (ADR4984), and _cdc16-1 cdc55_Δ (ADR5020) cells were arrested in G1 with 100 ng/ml α-factor. 1 h before α-factor wash-out, cells were shifted to 34 or 37°C to inactivate Cdc16. Cells were then washed and released from G1 arrest (t = 0) at either 34 or 37°C. Samples for fluorescence microscopy were taken at the indicated time points and the distance between Spc42-eGFP–labeled SPBs was measured in at least 100 cells. Anaphase spindles were defined as spindles >3 µm in length.
Figure 7.
PP2ACdc55-dependent dephosphorylation of Cdk1 sites on the APC inhibits anaphase onset. (A and B) Mutation of 12 Cdk1 phosphorylation sites on the APC in morphogenesis checkpoint-activated cells blocks _cdc55_Δ-dependent activation of the APC. _cdc55_Δ (ADR4738), _cdc55_Δ apc-12A (ADR4902), and apc-12A (ADR4973) cells were grown and treated with latA as in Fig. 3 A and samples for immunoblotting (A) and fluorescence microscopy (B) were taken at the indicated time points. More than 200 cells were scored for each data point in B. The data shown are from one representative experiment out of two repeats. (C and D) Mutation of 12 Cdk1 phosphorylation sites on the APC in spindle checkpoint-activated cells blocks _cdc55Δ_-dependent activation of the APC and rapid loss of viability. Wild-type (ADR4009), _mad2_Δ (ADR4099), _cdc55_Δ (ADR4738), _cdc55_Δ apc-12A (ADR4902), and apc-12A (ADR4973) cells were arrested in G1 with 100 ng/ml α-factor, released from G1, 10 µg/ml nocodazole was added at t = 35, and 800 ng/ml α-factor was re-added at t = 65 to arrest cells in the following G1. Samples for immunoblotting (C) and viability assays (D) were taken at the indicated time points. Viability (average ± SEM) was calculated from three independent experiments relative to viability at t = 0.
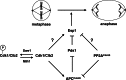
Figure 8.
Cdk1 activates and PP2ACdc55 inhibits anaphase onset. Our data support a model in which Cdk1 and PP2ACdc55 regulate phosphorylation of the APC and Esp1. In this model, Cdk1 regulates Esp1 directly by phosphorylation, and indirectly through modulation of APCCdc20 activity and Pds1 degradation. Dephosphorylation of tyrosine 19 on Cdk1 by the Mih1 (Cdc25) phosphatase allows normal anaphase onset by activating Cdk1, whereas Swe1 (Wee1) phosphorylation slows anaphase onset by phosphorylating and inhibiting Cdk1. During a morphogenesis checkpoint arrest, Swe1 is inhibited and Mih1 is activated, thereby blocking anaphase. Deletion of CDC55 allows premature activation of the APC and Esp1, which bypasses the morphogenesis checkpoint arrest despite continued signaling through inhibitory phosphorylation of Cdk1. Premature activation of the APC and Esp1 in cdc55Δ mutants could also explain cdc55Δ bypass of the spindle and DNA damage checkpoints, which block anaphase by inhibiting Cdc20 activity and Pds1 destruction, respectively (Minshull et al., 1996; Wang and Burke, 1997; Hwang et al., 1998; Kim et al., 1998; Sanchez et al., 1999; Tang and Wang, 2006).
Similar articles
- Redundant Regulation of Cdk1 Tyrosine Dephosphorylation in Saccharomyces cerevisiae.
Kennedy EK, Dysart M, Lianga N, Williams EC, Pilon S, Doré C, Deneault JS, Rudner AD. Kennedy EK, et al. Genetics. 2016 Mar;202(3):903-10. doi: 10.1534/genetics.115.182469. Epub 2015 Dec 29. Genetics. 2016. PMID: 26715668 Free PMC article. - Protein phosphatase 2A (PP2A) promotes anaphase entry after DNA replication stress in budding yeast.
Haluska C, Jin F, Wang Y. Haluska C, et al. Mol Biol Cell. 2021 Dec 1;32(22):ar36. doi: 10.1091/mbc.E21-04-0222. Epub 2021 Oct 20. Mol Biol Cell. 2021. PMID: 34668760 Free PMC article. - A phosphatase threshold sets the level of Cdk1 activity in early mitosis in budding yeast.
Harvey SL, Enciso G, Dephoure N, Gygi SP, Gunawardena J, Kellogg DR. Harvey SL, et al. Mol Biol Cell. 2011 Oct;22(19):3595-608. doi: 10.1091/mbc.E11-04-0340. Epub 2011 Aug 17. Mol Biol Cell. 2011. PMID: 21849476 Free PMC article. - The 'anaphase problem': how to disable the mitotic checkpoint when sisters split.
Vázquez-Novelle MD, Mirchenko L, Uhlmann F, Petronczki M. Vázquez-Novelle MD, et al. Biochem Soc Trans. 2010 Dec;38(6):1660-6. doi: 10.1042/BST0381660. Biochem Soc Trans. 2010. PMID: 21118144 Review. - The spindle checkpoint: how do cells delay anaphase onset?
Sczaniecka MM, Hardwick KG. Sczaniecka MM, et al. SEB Exp Biol Ser. 2008;59:243-56. SEB Exp Biol Ser. 2008. PMID: 18368927 Review.
Cited by
- Adaptation to the spindle checkpoint is regulated by the interplay between Cdc28/Clbs and PP2ACdc55.
Vernieri C, Chiroli E, Francia V, Gross F, Ciliberto A. Vernieri C, et al. J Cell Biol. 2013 Sep 2;202(5):765-78. doi: 10.1083/jcb.201303033. J Cell Biol. 2013. PMID: 23999167 Free PMC article. - A Conserved PP2A Regulatory Subunit Enforces Proportional Relationships Between Cell Size and Growth Rate.
Leitao RM, Jasani A, Talavara RA, Pham A, Okobi QJ, Kellogg DR. Leitao RM, et al. Genetics. 2019 Oct;213(2):517-528. doi: 10.1534/genetics.119.301012. Epub 2019 Sep 5. Genetics. 2019. PMID: 31488515 Free PMC article. - Kar9 symmetry breaking alone is insufficient to ensure spindle alignment.
Meziane M, Genthial R, Vogel J. Meziane M, et al. Sci Rep. 2021 Feb 19;11(1):4227. doi: 10.1038/s41598-021-83136-w. Sci Rep. 2021. PMID: 33608583 Free PMC article. - Phosphate-binding pocket on cyclin B governs CDK substrate phosphorylation and mitotic timing.
Ng HY, Whelpley DH, Adly AN, Maxwell RA, Morgan DO. Ng HY, et al. Nat Commun. 2025 May 8;16(1):4281. doi: 10.1038/s41467-025-59700-7. Nat Commun. 2025. PMID: 40341598 Free PMC article. - An interaction between myosin-10 and the cell cycle regulator Wee1 links spindle dynamics to mitotic progression in epithelia.
Sandquist JC, Larson ME, Woolner S, Ding Z, Bement WM. Sandquist JC, et al. J Cell Biol. 2018 Mar 5;217(3):849-859. doi: 10.1083/jcb.201708072. Epub 2018 Jan 10. J Cell Biol. 2018. PMID: 29321170 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous