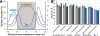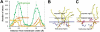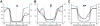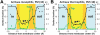Structural adaptations of proteins to different biological membranes - PubMed (original) (raw)
Structural adaptations of proteins to different biological membranes
Irina D Pogozheva et al. Biochim Biophys Acta. 2013 Nov.
Abstract
To gain insight into adaptations of proteins to their membranes, intrinsic hydrophobic thicknesses, distributions of different chemical groups and profiles of hydrogen-bonding capacities (α and β) and the dipolarity/polarizability parameter (π*) were calculated for lipid-facing surfaces of 460 integral α-helical, β-barrel and peripheral proteins from eight types of biomembranes. For comparison, polarity profiles were also calculated for ten artificial lipid bilayers that have been previously studied by neutron and X-ray scattering. Estimated hydrophobic thicknesses are 30-31Å for proteins from endoplasmic reticulum, thylakoid, and various bacterial plasma membranes, but differ for proteins from outer bacterial, inner mitochondrial and eukaryotic plasma membranes (23.9, 28.6 and 33.5Å, respectively). Protein and lipid polarity parameters abruptly change in the lipid carbonyl zone that matches the calculated hydrophobic boundaries. Maxima of positively charged protein groups correspond to the location of lipid phosphates at 20-22Å distances from the membrane center. Locations of Tyr atoms coincide with hydrophobic boundaries, while distributions maxima of Trp rings are shifted by 3-4Å toward the membrane center. Distributions of Trp atoms indicate the presence of two 5-8Å-wide midpolar regions with intermediate π* values within the hydrocarbon core, whose size and symmetry depend on the lipid composition of membrane leaflets. Midpolar regions are especially asymmetric in outer bacterial membranes and cell membranes of mesophilic but not hyperthermophilic archaebacteria, indicating the larger width of the central nonpolar region in the later case. In artificial lipid bilayers, midpolar regions are observed up to the level of acyl chain double bonds.
Keywords: Hydrophobic thickness; Hyperthermophile; Lipid bilayer; Membrane asymmetry; Membrane protein; Polarity profile.
© 2013.
Figures
Figure 1
Different types of integral membrane proteins positioned in membranes: TM α-helical proteins, single-chain β-barrels (β-I type) and multi-chain β-barrels (β-II type). Lipid-facing residues are shown by colored spheres: Lys and Arg (blue), Asp and Glu (red), Tyr (purple), Trp (green). Co-crystallized lipids are shown by sticks colored orange (C and P atoms), red (O-atoms) and blue (N-atoms). Hydrophobic boundaries calculated by PPM are shown by horizontal lines: blue (for PM cytoplasmic side or OM periplasmic side) and red (for PM extracellular side or IM periplasmic side). Cartoon representations of proteins are colored by chain. All selected proteins represent oligomers: homotrimer of sucrose-specific porin, heterodimer of BtuB cobalamin transporter, homotrimer of drug discharge proteins OprM, homotrimer of bacteriorhodopsin, homotetramer of aquaporin-0. Trimerization of OrpM is required to form TM β-barrel. Distributions of Tyr, Trp, Lys, Arg, Asp, and Glu on the surface of membrane proteins are clearly nonuniform with charged residues accumulated in the lipid headgroup regions, and Trp and Tyr residues located near hydrophobic boundaries inside and outside of the hydrocarbon region, respectively.
Figure 2
Structures of DOPC (A) and POPG (B) bilayers. Distributions of lipid segments determined by the simulteneous analysis of X-ray and neutron scattering data (left panels). Volume probability distributions of various lipid component were determined assuming that the total probability is equal to 1 at each point across the bilayer. Changes of polarity parameters, hydrogen bonding donor (α), acceptor (β) capacities and dipolarity/polarizability paramere (π*) along the membrane normal (right panels). Structural parameters are indicated by arrows: hydrophobic thickness (2_DC_), distance between lipid head groups (DHH), total bilayer thickness (hydrocarbon chains plus head groups) (DB). Parameters used for calculation of these profiles and references are provided in Tables 2, S3 and S4.
Figure 3
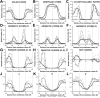
(A-I) Distributions of lipid-facing protein atoms in structures of TM α-helical protein from six membrane types: eukaryotic PM (50 structures, blue line), PM of Gram-positive bacteria (12 proteins, purple line), PM of archaeabacteria (20 structures, yellow line), IM of Gram-negative bacteria (82 structures, red line), IM of mitochondria (9 structures, black line), thylakoid membrane (8 structures, green line). Polar atoms are N- and O-atoms; nonpolar atoms are C- and S-atoms from side chains of Val, Leu, Ile, Met, Cys, Phe, Tyr, and Trp residues. Aromatic atoms are C-atoms from benzene rings of Tyr and Phe and from indole ring of Trp. Charged groups are: amine group of Lys, guanidinium group of Arg, carboxyl group of Asp and Glu. (J-L) Transbilayer profies of polarity parameters: hydrogen bonding donor (α) and acceptor (β) capacities and solvatochromic dipolarity/polarisability parameter (π*). Similarities in distributions and polarity profiles are observed inside the hydrophobic boundaries (±15 Å from the membrane center), while most differences are seen outside these boundaries.
Figure 4
(A-I) Comparison of distributions of lipid-facing protein atoms in structures of 191 TM α-helical protein from six membrane types (solid black line), 68 single-chain TM β-barrels (β-I type, solid grey line), and 5 multi-chain TM β-barrels (β-II type, dashed grey line). Distribution across the membrane were analyzed for polar atoms (N- and O-atoms of main and side chains), nonpolar atoms (C- and S-atoms from side chains of Val, Leu, Ile, Met, Cys, Phe, Tyr, Trp), aromatic atoms (C-atoms from benzene rings of Tyr, Phe and indole ring of Trp), and charged groups (amine group of Lys, guanidinium group of Arg, carboxyl group of Asp and Glu). (J-L) Comparison of transbilayer profiles of corresponding polarity parameters (α, β, π*). Differences in atom distributions and in polarity profiles for α-helical and β-barrel proteins are observed inside and outside the hydrophobic boundaries. These boundaries for α-barrels are shifted by 3-4 Å toward the membrane center as compared to α-helical proteins, indicating the smaller hydrophobic thickness of TM β-barrels. The distributions of charged residues and net charges are similar for α-helical and β-II proteins, but different for β-I proteins.
Figure 5
Comparison of calculated hydrophobic thicknesses (grey) with midpoints of distributions of polar atoms (light blue), nonpolar atoms (brown), co-crystallized water (dark blue), and maxima of distribution of Tyr atoms (purple) in 191 selected TM α-helical proteins and protein complexes (A). Comparison of calculated hydrophobic thicknesses (grey) with distances between midpoints of distributions of polar atoms (light blue), nonpolar atoms (brown), and co-crystallized water (dark blue), and with distances between maxima of Tyr distributions (B). Analysis was performed for TM β-barrels from OM of Gram-negative bacteria and TM α-helical proteins from six membrane types: PM of eukaryotic cells, Gram-positive bacteria, archaeabacteria, thylakoid membranes, IM of mitochondria, and Gram-negative bacteria. Numbers of protein structures in each set are indicated in parenthesis. Calculated hydrophobic boundaries match the positions of midpoints of distribution curves of polar, nonpolar atoms and co-crystallized water, as well as maxima of Tyr distributions.
Figure 6
Distribution of different groups of lipids co-crystallyzed with 164 TM α-helical proteins: ester and ether oxygens (green line and dots), phosphates (orange line and diamonds) and head group atoms (yellow line and squares) (A). Right pictures show position of 1,2-stearoyl-_sn_-glycero-3-phosphatidylethanolamine co-crystallized with bacterial cytochrome C oxidase (1M56) (B) and position of 2,3-Di-O-Phytanly-3-_sn_-Glycero-1-Phosphoryl-3′-_sn_-Glycerol-1′-Phosphate co-crystallized with bacteriorhodopsin (1IW6) (C) relative to the hydrophobic protein boundaries calculated by PPM (marked by blue and red lines). Lipid molecules are colored yellow (C atoms), red (O-atoms), and orange (P-atoms). Arg and Lys residues are colored blue (C-atoms), dark blue (N-atoms). Tyr residues are colored purple (C-atoms) and red (O-atoms). Hydrogen bonds between lipid head groups and Arg residues are indicated by black dashes.
Figure 7
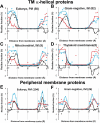
Distributions of lipid-facing charged groups of Lys and Arg (blue lines and squares), Asp and Glu (red lines and triangles) and net charges (black lines and rhombs) in structures of TM α-helical proteins (A-D) and peripheral membrane proteins (E-F) from different membrane types: eukaryotic PM (A,E), IM of Gram-negative bacteria (B,F), IM of mitochondria (C), and thylakoid membranes (D). Numbers of protein structures in each set are indicated in parenthesis.
Figure 8
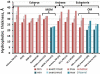
Intrinsic hydrophobic thickness of membrane proteins from nine membrane types: PM of eukaryotic cell, Gram-positive (G(+)) bacteria, and archaeabacteria, endoplasmic reticulum (ER) membranes, thylakoid membranes, mitochondrial IM and OM (MIM, MOM) and Gram-negative bacteria (G(-)). Numbers of protein structures in each set are indicated in parenthesis.
Figure 9
Comparison of transbilayer profiles of polarity parameters (α, β, and π*) calculated for artificial lipid bilayer, DOPC (red lines), POPG (blue lines), and for lipid-facing atoms of TM α-helical proteins from IM of Gram-negative bacteria (82 structures, black solid lines) and from PM of eukaryotic cells (34 structures, black dashed lines). In each panel vertical axis corresponds to a polarity parameter calculated for artificial lipid bilayers (left axis) and TM proteins (right axis).
Figure 10
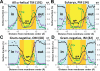
Localization of midpolar regions based on distributions of lipid-facing atoms of TM α-helical proteins. (A) Analysis of distributions of polar atoms, N and O (blue line), Tyr atoms (purple line), Trp atoms (green line), and polarity parameter π* calculated for 191 structures of TM α-helical proteins. Midpolar regions are colored orange, head group regions are colored light blue, central nonpolar regions are colored yellow. (B) Comparison of polarity profile of parameter π* calculated for 34 structures of TM α-helical proteins from eukaryotic PM (blue line and squares) and 82 structures of TM α-helical proteins from IM of Gram-negative bacteria.
Figure 11
Comparison of the fine structure of the hydrocarbon core region in mesophilic and thermophilic archaeabacteria. Localization of the midpolar regions is based on the distributions of lipid-facing Trp atoms and polarity parameter π* calculated for structures of TM α-helical proteins from mesophilic (A) and thermophilic (B) archaebacteria. Numbers of protein structures in each set are indicated in parenthesis. Midpolar regions are colored orange, head group regions are colored light blue, central nonpolar regions are colored yellow.
Similar articles
- Life at the border: adaptation of proteins to anisotropic membrane environment.
Pogozheva ID, Mosberg HI, Lomize AL. Pogozheva ID, et al. Protein Sci. 2014 Sep;23(9):1165-96. doi: 10.1002/pro.2508. Epub 2014 Jul 2. Protein Sci. 2014. PMID: 24947665 Free PMC article. Review. - Anisotropic solvent model of the lipid bilayer. 2. Energetics of insertion of small molecules, peptides, and proteins in membranes.
Lomize AL, Pogozheva ID, Mosberg HI. Lomize AL, et al. J Chem Inf Model. 2011 Apr 25;51(4):930-46. doi: 10.1021/ci200020k. Epub 2011 Mar 25. J Chem Inf Model. 2011. PMID: 21438606 Free PMC article. - Positioning of proteins in membranes: a computational approach.
Lomize AL, Pogozheva ID, Lomize MA, Mosberg HI. Lomize AL, et al. Protein Sci. 2006 Jun;15(6):1318-33. doi: 10.1110/ps.062126106. Protein Sci. 2006. PMID: 16731967 Free PMC article. - Bilayer hydrophobic thickness and integral membrane protein function.
Cybulski LE, de Mendoza D. Cybulski LE, et al. Curr Protein Pept Sci. 2011 Dec;12(8):760-6. doi: 10.2174/138920311798841681. Curr Protein Pept Sci. 2011. PMID: 22044142 Review.
Cited by
- Plant membrane assays with cytokinin receptors underpin the unique role of free cytokinin bases as biologically active ligands.
Lomin SN, Krivosheev DM, Steklov MY, Arkhipov DV, Osolodkin DI, Schmülling T, Romanov GA. Lomin SN, et al. J Exp Bot. 2015 Apr;66(7):1851-63. doi: 10.1093/jxb/eru522. Epub 2015 Jan 21. J Exp Bot. 2015. PMID: 25609827 Free PMC article. - MemSTATS: A Benchmark Set of Membrane Protein Symmetries and Pseudosymmetries.
Aleksandrova AA, Sarti E, Forrest LR. Aleksandrova AA, et al. J Mol Biol. 2020 Jan 17;432(2):597-604. doi: 10.1016/j.jmb.2019.09.020. Epub 2019 Oct 16. J Mol Biol. 2020. PMID: 31628944 Free PMC article. - Identification and assessment of cardiolipin interactions with E. coli inner membrane proteins.
Corey RA, Song W, Duncan AL, Ansell TB, Sansom MSP, Stansfeld PJ. Corey RA, et al. Sci Adv. 2021 Aug 20;7(34):eabh2217. doi: 10.1126/sciadv.abh2217. Print 2021 Aug. Sci Adv. 2021. PMID: 34417182 Free PMC article. - Open Questions on the Origin of Eukaryotes.
López-García P, Moreira D. López-García P, et al. Trends Ecol Evol. 2015 Nov;30(11):697-708. doi: 10.1016/j.tree.2015.09.005. Epub 2015 Oct 8. Trends Ecol Evol. 2015. PMID: 26455774 Free PMC article. Review. - Using Alphafold2 to Predict the Structure of the Gp5/M Dimer of Porcine Respiratory and Reproductive Syndrome Virus.
Veit M, Gadalla MR, Zhang M. Veit M, et al. Int J Mol Sci. 2022 Oct 30;23(21):13209. doi: 10.3390/ijms232113209. Int J Mol Sci. 2022. PMID: 36361998 Free PMC article.
References
- Findlay HE, Booth PJ. The biological significance of lipid-protein interactions. J. Phys.: Condens. Matter. 2006;18:S1281–S1291. - PubMed
- Epand RM. Lipid polymorphism and protein-lipid interactions. Biochim. Biophys. Acta Biomembr. 1998;1376:353–368. - PubMed
- MacCallum JL, Tieleman DP. Interactions between small molecules and lipid bilayers. Curr. Top. Memb. 2008;60:227–256.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous