Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury - PubMed (original) (raw)
. 2013 Jul 16;110(29):12024-9.
doi: 10.1073/pnas.1305538110. Epub 2013 Jul 1.
Jan Hinrich Bräsen, Maurice Darding, Mi Kyung Jin, Ana B Sanz, Jan-Ole Heller, Federica De Zen, Ricardo Weinlich, Alberto Ortiz, Henning Walczak, Joel M Weinberg, Douglas R Green, Ulrich Kunzendorf, Stefan Krautwald
Affiliations
- PMID: 23818611
- PMCID: PMC3718149
- DOI: 10.1073/pnas.1305538110
Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury
Andreas Linkermann et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Regulated necrosis (RN) may result from cyclophilin (Cyp)D-mediated mitochondrial permeability transition (MPT) and receptor-interacting protein kinase (RIPK)1-mediated necroptosis, but it is currently unclear whether there is one common pathway in which CypD and RIPK1 act in or whether separate RN pathways exist. Here, we demonstrate that necroptosis in ischemia-reperfusion injury (IRI) in mice occurs as primary organ damage, independent of the immune system, and that mice deficient for RIPK3, the essential downstream partner of RIPK1 in necroptosis, are protected from IRI. Protection of RIPK3-knockout mice was significantly stronger than of CypD-deficient mice. Mechanistically, in vivo analysis of cisplatin-induced acute kidney injury and hyperacute TNF-shock models in mice suggested the distinctness of CypD-mediated MPT from RIPK1/RIPK3-mediated necroptosis. We, therefore, generated CypD-RIPK3 double-deficient mice that are viable and fertile without an overt phenotype and that survived prolonged IRI, which was lethal to each single knockout. Combined application of the RIPK1 inhibitor necrostatin-1 and the MPT inhibitor sanglifehrin A confirmed the results with mutant mice. The data demonstrate the pathophysiological coexistence and corelevance of two separate pathways of RN in IRI and suggest that combination therapy targeting distinct RN pathways can be beneficial in the treatment of ischemic injury.
Keywords: RIP1; RIP3; apoptosis; programmed necrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
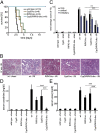
Fig. 1.
Increased protection from ischemia–reperfusion damage by combined loss of RIPK3 and CypD. Mice underwent severe renal IRI. (A) Survival proportions of indicated mice following IRI. (B) Representative periodic acid–Schiff (PAS)-stained histomicrographs of mice with indicated genotype 48 h after severe IRI. White arrows point to typical necrotic changes classically observed in proximal tubules upon renal IRI. (C) Quantification by renal damage score of B. (D and E) Serum creatinine and serum urea concentrations 48 h following reperfusion or sham operation. **P < 0.01; ***P < 0.001 (n = 8–12 per group).
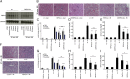
Fig. 2.
RIPK3 and CypD contribute to ischemia–reperfusion damage but caspase-8 does not. (A) Expression levels of RIPK1 in whole-kidney lysates taken from wt or RIPK3-ko mice during the time course of IRI in wt mice. GAPDH serves as a loading control. (B) wt, RIPK3-ko, and caspase-8 (C8)/RIPK3-dko mice underwent renal IRI 48 h before preparation of PAS-stained renal sections and its quantification using the renal damage score (C). (D and E) Corresponding serum creatinine and serum urea concentrations 48 h after reperfusion (n = 8–12 per group). (F–I) Comparison of wt, CypD-ko, and RIPK3-ko mice in a mild IRI setting (n = 7–16 per group).
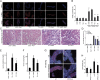
Fig. 3.
Necroptosis occurs independently of the immune system. (A) Freshly isolated renal tubules were treated for 6 h after preparation with indicated agents before assessment of TUNEL positivity and quantification (B). (C and D) Representative PAS-stained kidney sections (C) and evaluation of renal damage (D) from SCID-Beige mice that underwent ischemia–reperfusion in the presence of DMSO or Nec-1. Corresponding serum concentrations of creatinine (E) and urea (F) are shown 48 h after reperfusion (n = 8 per group). (G) PI staining of freshly isolated renal tubules from wt or RIPK3-ko mice that underwent 60 min of hypoxia followed by 60 min of reperfusion. (H) LDH-release assay of the similar tubules as in G.
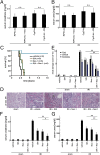
Fig. 4.
Combination therapy with Nec-1 and SfA provides strong protection against IRI. (A and B) RIPK3-ko and CypD-ko mice (n = 8 per group) underwent indicated treatment 15 min before onset of IRI surgery. Note that the neither the addition of Nec-1 to RIPK3-ko mice nor the application of SfA in CypD-ko mice led to any further protection compared with the DMSO-treated knockouts. (C–G) wt mice (n = 8–12 per group) underwent indicated treatment 15 min before the onset of surgery. (C) Survival proportions following IRI. (D) Representative PAS-stained histomicrographs 48 h after severe IRI are demonstrated. (E) Quantification by renal damage score of D. (F–G) Serum creatinine and serum urea concentrations 48 h following reperfusion or sham operation. Note the additive protective effect of combination therapy. *P < 0.05; **P < 0.01; n.s., not significant.
Similar articles
- Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury.
Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J, Hu S, Chen Y, Zhang Y. Zhou H, et al. J Pineal Res. 2018 Oct;65(3):e12503. doi: 10.1111/jpi.12503. Epub 2018 May 28. J Pineal Res. 2018. PMID: 29770487 - Cyclophilin D-mediated regulation of the permeability transition pore is altered in mice lacking the mitochondrial calcium uniporter.
Parks RJ, Menazza S, Holmström KM, Amanakis G, Fergusson M, Ma H, Aponte AM, Bernardi P, Finkel T, Murphy E. Parks RJ, et al. Cardiovasc Res. 2019 Feb 1;115(2):385-394. doi: 10.1093/cvr/cvy218. Cardiovasc Res. 2019. PMID: 30165576 Free PMC article. - Programmed necrosis in heart disease: Molecular mechanisms and clinical implications.
Zhu H, Sun A. Zhu H, et al. J Mol Cell Cardiol. 2018 Mar;116:125-134. doi: 10.1016/j.yjmcc.2018.01.018. Epub 2018 Feb 6. J Mol Cell Cardiol. 2018. PMID: 29426003 Review. - Regulated necrosis in kidney ischemia-reperfusion injury.
Pefanis A, Ierino FL, Murphy JM, Cowan PJ. Pefanis A, et al. Kidney Int. 2019 Aug;96(2):291-301. doi: 10.1016/j.kint.2019.02.009. Epub 2019 Mar 7. Kidney Int. 2019. PMID: 31005270 Review.
Cited by
- Regulated necrosis role in inflammation and repair in acute kidney injury.
Guerrero-Mauvecin J, Villar-Gómez N, Rayego-Mateos S, Ramos AM, Ruiz-Ortega M, Ortiz A, Sanz AB. Guerrero-Mauvecin J, et al. Front Immunol. 2023 Nov 24;14:1324996. doi: 10.3389/fimmu.2023.1324996. eCollection 2023. Front Immunol. 2023. PMID: 38077379 Free PMC article. Review. - A phase I randomized study to evaluate safety, pharmacokinetics, and pharmacodynamics of SIR2446M, a selective RIPK1 inhibitor, in healthy participants.
Sun ALA, Gillies JD, Shen Y, Deng H, Xue F, Ma Y, Song L. Sun ALA, et al. Clin Transl Sci. 2024 Jul;17(7):e13857. doi: 10.1111/cts.13857. Clin Transl Sci. 2024. PMID: 38949195 Free PMC article. Clinical Trial. - MicroRNA-223-5p and -3p Cooperatively Suppress Necroptosis in Ischemic/Reperfused Hearts.
Qin D, Wang X, Li Y, Yang L, Wang R, Peng J, Essandoh K, Mu X, Peng T, Han Q, Yu KJ, Fan GC. Qin D, et al. J Biol Chem. 2016 Sep 16;291(38):20247-59. doi: 10.1074/jbc.M116.732735. Epub 2016 Aug 8. J Biol Chem. 2016. PMID: 27502281 Free PMC article. - Phosphorylated MLKL causes plasma membrane rupture.
Linkermann A, Kunzendorf U, Krautwald S. Linkermann A, et al. Mol Cell Oncol. 2014 Aug 13;1(1):e29915. doi: 10.4161/mco.29915. eCollection 2014. Mol Cell Oncol. 2014. PMID: 27308322 Free PMC article. No abstract available. - Silencing lncRNA KCNQ1OT1 reduced hepatic ischemia reperfusion injury-induced pyroptosis by regulating miR-142a-3p/HMGB1 axis.
Liang C, Peng Y, Sun H, Wang L, Jiang L, Zou S. Liang C, et al. Mol Cell Biochem. 2023 Jun;478(6):1293-1305. doi: 10.1007/s11010-022-04586-y. Epub 2022 Oct 29. Mol Cell Biochem. 2023. PMID: 36308669
References
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. - PubMed
- Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. - PubMed
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14(8):2199–2210. - PubMed
- Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK34275/DK/NIDDK NIH HHS/United States
- R56 DK034275/DK/NIDDK NIH HHS/United States
- R01 CA169291/CA/NCI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- R01 AI044828/AI/NIAID NIH HHS/United States
- R01 DK034275/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous