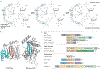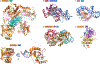Breaking and joining single-stranded DNA: the HUH endonuclease superfamily - PubMed (original) (raw)
Review
Breaking and joining single-stranded DNA: the HUH endonuclease superfamily
Michael Chandler et al. Nat Rev Microbiol. 2013 Aug.
Abstract
HUH endonucleases are numerous and widespread in all three domains of life. The major function of these enzymes is processing a range of mobile genetic elements by catalysing cleavage and rejoining of single-stranded DNA using an active-site Tyr residue to make a transient 5'-phosphotyrosine bond with the DNA substrate. These enzymes have a key role in rolling-circle replication of plasmids and bacteriophages, in plasmid transfer, in the replication of several eukaryotic viruses and in various types of transposition. They have also been appropriated for cellular processes such as intron homing and the processing of bacterial repeated extragenic palindromes. Here, we provide an overview of these fascinating enzymes and their functions, using well-characterized examples of Rep proteins, relaxases and transposases, and we explore the molecular mechanisms used in their diverse activities.
Conflict of interest statement
Competing interests statement
The authors declare no competing financial interests.
Figures
Figure 1 |. Reaction mechanism and organization of selected HUH proteins.
a | The HUH endonuclease domain (in which U is a hydrophobic residue), together with the divalent metal ion (M2+) ligand, nicks single-stranded DNA (ssDNA) to form a covalent intermediate and release the cleaved leaving group. b | Both the Rep (replication) domain of adeno-associated virus 5 (AAV5) Rep (Protein Data Bank (PDB) accession 1M55) and the relaxase domain of plasmid R388 TrwC (PDB accession 1OMH) have a five-stranded β-sheet with a βαββ core that harbours the HUH motif on the central strand. Owing to the different domain organization in Rep proteins and relaxases, the position of this core differs (βαββαβαβ for Rep proteins and βαβαβαββ for relaxases). The more amino-terminal residues in the primary sequence are shown in pink; those more carboxy-terminal are in blue. c | The organization of representative HUH domain-containing proteins is shown; they contain HUH, helicase, oligomerization (OD) and proposed Zn-binding (not necessarily structurally related) domains. The length of each protein is indicated in numbers of amino acids, and those proteins for which HUH domain structures are available are indicated with an asterisk; the HUH motif data are taken from REF. (motif 2) and REF. (motif III); the Y motif data are taken from REF. (motif 3) and REF. (motif I). The assigned domain organizations are taken from phage φXl74 protein A (gpA), AAV Rep78 (REF. 102), tomato yellow leaf curl virus (TYLCV) Rep, plasmid pMV158 RepB, plasmid R388 TrwC, plasmid RSF1010 MobA (mobilization protein A), transposases from the insertion sequences IS608 (REF. 7), IS_91_ (REF. 7) and insertion sequence with a common region 1 (ISCR1) (S. Messing, A.B.H. and F.D., unpublished observations), and HeliBat1 (a consensus sequence from a bioinformatic prediction). Image in part a is modified, with permission, from REF. © (2005) Cell Press.
Figure 2 |. Structures of various HUH enzymes with their substrate DNAs.
a | The interaction of adeno-associated virus (AAV) Rep (replication) protein with the inverted terminal repeat hairpin in the DNA; the top panel shows the interaction with the 20 bp Rep-binding site (RBS) (Protein Data Bank (PDB) accession 1RZ9), and the lower panel shows the interaction with the Rep-binding element (RBE’) (PDB accession 1UUT). b | The interaction between the plasmid R388 relaxase, TrwC, and a 25-base oligonucleotide containing both the plasmid nic site and the recognition hairpin (PDB accession 2CDM). c | The interaction of nicking enzyme in Staphyloeoeeus (NES) with the origin of transfer (oriT), represented by an oligonucleotide of 30 bases (PDB accession 4HT4). d | A model of the interaction between insertion sequence IS_6_08 transposase (TnpA) and both the transposon left end (LE) (PDB accession 2VJV) and right end (RE) (PDB accession 2VHG). e | The interaction of TnpA(REP) with a repetitive extragenic palindromic (REP) sequence (PDB accession 4ER8).
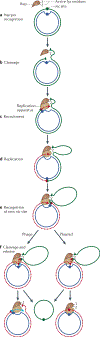
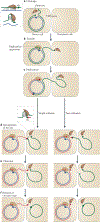
Figure 3 |. Rolling-circle replication.
a | The Rep (replication) protein contains two active-site Tyr residues, 1 and 2, and recognizes circular double-stranded DNA (positive-sense strand in green, negative-sense strand in blue) at the the 3′ recognition sequence. b | Rep cleaves the DNA at the_nic_site and forms a phosphotyrosine bond with Tyr residue 1. c | The replication apparatus is recruited. d | Replication is initiated and reconstitutes the_nic_site. e | When the entire circle of DNA has been replicated, Rep and the replication apparatus reach the reconstituted_nic_site. f | In the case of phage replication, the_nic_site is cleaved by the Rep active-site Tyr residue 2 to form a new phosphotyrosine. The resulting 3′-OH attacks the original phosphotyrosine bond (with Tyr residue 1) to release a single-stranded circular phage copy. Replication of the Rep-bound circular DNA then continues round the circle again. In the case of copy number-regulated plasmid replication, no second phosphotyrosine bond is formed, and the plasmid regains its initial circular dsDNA form possibly through the use of a second nucleophile (such as water) instead of a Tyr. Failure to terminate at this stage would lead to multimeric single-stranded products.
Figure 4 |. Rolling hairpin replication of adeno-associated virus.
For simplicity, secondary structures at the ends of the single-stranded adeno-associated virus (AAV) genome (called inverted terminal repeats (ITRs)) are shown as T junctions with complementary base pairing. Parental DNA is green and newly synthesized DNA is red. a | The insert shows a schematic organization of the ITR, and blue circles indicate ITR regions that interact with the protein Rep (replication). Replication is initiated using the host replication machinery and the 3′-OH of the 3′ ITR as a primer. b,c | Replication continues to the end of the genome, duplicating the 5′-terminal ITR structure. d | For AAV5, five Rep molecules bind to the 20-base (GCTC)5 Rep-binding site (RBS) in the 3′ ITR and contact the Repbinding element (RBE′) hairpin tip. e | This binding is proposed to provoke a conformational change in the DNA at the palindromic terminal resolution site (trs), and to induce Rep-mediated cleavage at trs to generate a phosphotyrosine bond between Rep and the DNA. f | Recruitment of the host replication machinery then allows replication of the 3′-terminal structure (a step called terminal resolution). g | Refolding of the ends generates structures resembling those that are present before replication initiation. h | The end result is a fully replicated viral genome.
Figure 5 |. Rolling-circle replication-mediated conjugation.
a | Conjugative transfer occurs through a type IV secretion system (T4SS) pore between the donor and recipient cells. Conjugation is initiated by the relaxase recognizing one side of a double-stranded inverted repeat structure (insert) proximal to a 3′ nic site. Nucleophilic attack of the nic site generates a phosphotyrosine bond between the cleaved strand of the nic site and a Tyr residue in the relaxase. b | The linked single-stranded DNA (ssDNA) is transferred with the relaxase into the recipient cell. c | Replication is initiated in the donor cell using the host replication apparatus and regenerates the nic site. d-f | Replication continues until the entire circle of DNA has been replicated. The nic site that was reconstituted at the onset of replication is then cleaved by a relaxase (insert) to generate a circular ssDNA copy of the genome in the recipient cell. This cleavage can be mediated by the relaxase bound to the original nicked end, in the recipient cell, or by a second relaxase, in the donor cell. Replication in the donor cell is generally concomitant with transfer but is not essential. Replication of the transferred single strand is carried out by the replication apparatus of the recipient cell.
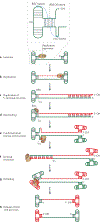
Figure 6 |. Single-strand transposons.
a | The genetic organization of the insertion sequence IS_608_, a member of the IS_200_-IS_605_ family. The ORFs tnpA and tnpB encode transposase (TnpA; a Y1 transposase) and a protein of unknown function, TnpB. The left end (LE) and right end (RE) regions have potential secondary structures, as indicated schematically. The left and right guide sequences (GL and GR, respectively) recognize and interact with the left and right cleavage sites
(Cl
and CR, respectively). b | IS_200_-IS_605_ transposons preferentially excise from and integrate into the lagging-strand template of the replication fork. The dashed red line represents an Okazaki fragment, and the solid red line represents the newly synthesized leading strand. The dimeric TnpA (black sticks indicating catalytic Tyr residues) catalyses excision of the single-stranded insertion sequence, resulting in a deletion in the lagging-stand template. The single-stranded insertion sequence then attacks the lagging-strand template in a target molecule and inserts 3′ to the TTAC target tetranucleotide. c | Each monomer of the IS_608_ TnpA dimer undergoes a series of cis-trans conformational changes during the transposition cycle, displacing the HUH motif (in which U is a hydrophobic residue) in the main body of the protein and the catalytic Tyr residue (Y) situated on an α-helix (αD). For binding and cleavage of the left and right ends of the insertion sequence, the active sites adopt a trans configuration, in which the HUH motif of an active site is contributed by one TnpA monomer and the Tyr residue is contributed by the other (step 1). Following DNA cleavage, the two αD-helices rotate to the cis configuration (step 2). In this configuration, the αD-helices then catalyse the formation of a circular single-stranded transposon and join the cleaved ends of the donor sequence (step 3). The configuration is then reset to the trans form to end excision (step 4). Integration starts with the active sites, in a trans configuration, cleaving the single-stranded transposon and the target DNA (step 5). The two αD-helices rotate for strand transfer (step 6), and the active sites return to a cis configuration to integrate the transposon (step 7).
Similar articles
- The secret life of conjugative relaxases.
Guzmán-Herrador DL, Llosa M. Guzmán-Herrador DL, et al. Plasmid. 2019 Jul;104:102415. doi: 10.1016/j.plasmid.2019.102415. Epub 2019 May 17. Plasmid. 2019. PMID: 31103521 Review. - Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria.
Ilyina TV, Koonin EV. Ilyina TV, et al. Nucleic Acids Res. 1992 Jul 11;20(13):3279-85. doi: 10.1093/nar/20.13.3279. Nucleic Acids Res. 1992. PMID: 1630899 Free PMC article. - Crystal structure of the Wheat dwarf virus Rep domain.
Everett BA, Litzau LA, Tompkins K, Shi K, Nelson A, Aihara H, Evans Iii RL, Gordon WR. Everett BA, et al. Acta Crystallogr F Struct Biol Commun. 2019 Dec 1;75(Pt 12):744-749. doi: 10.1107/S2053230X19015796. Epub 2019 Nov 27. Acta Crystallogr F Struct Biol Commun. 2019. PMID: 31797816 Free PMC article. - Computer-assisted dissection of rolling circle DNA replication.
Koonin EV, Ilyina TV. Koonin EV, et al. Biosystems. 1993;30(1-3):241-68. doi: 10.1016/0303-2647(93)90074-m. Biosystems. 1993. PMID: 8374079 - Bringing them together: plasmid pMV158 rolling circle replication and conjugation under an evolutionary perspective.
Lorenzo-Díaz F, Fernández-López C, Garcillán-Barcia MP, Espinosa M. Lorenzo-Díaz F, et al. Plasmid. 2014 Jul;74:15-31. doi: 10.1016/j.plasmid.2014.05.004. Epub 2014 Jun 2. Plasmid. 2014. PMID: 24942190 Free PMC article. Review.
Cited by
- Identification, Characterization, and Application of the Replicon Region of the Halophilic Temperate Sphaerolipovirus SNJ1.
Wang Y, Sima L, Lv J, Huang S, Liu Y, Wang J, Krupovic M, Chen X. Wang Y, et al. J Bacteriol. 2016 Jun 27;198(14):1952-1964. doi: 10.1128/JB.00131-16. Print 2016 Jul 15. J Bacteriol. 2016. PMID: 27137505 Free PMC article. - Bacterial insertion sequences: their genomic impact and diversity.
Siguier P, Gourbeyre E, Chandler M. Siguier P, et al. FEMS Microbiol Rev. 2014 Sep;38(5):865-91. doi: 10.1111/1574-6976.12067. Epub 2014 Feb 26. FEMS Microbiol Rev. 2014. PMID: 24499397 Free PMC article. Review. - Mriyaviruses: small relatives of giant viruses.
Yutin N, Mutz P, Krupovic M, Koonin EV. Yutin N, et al. mBio. 2024 Jul 17;15(7):e0103524. doi: 10.1128/mbio.01035-24. Epub 2024 Jun 4. mBio. 2024. PMID: 38832788 Free PMC article. - A Helitron transposon reconstructed from bats reveals a novel mechanism of genome shuffling in eukaryotes.
Grabundzija I, Messing SA, Thomas J, Cosby RL, Bilic I, Miskey C, Gogol-Döring A, Kapitonov V, Diem T, Dalda A, Jurka J, Pritham EJ, Dyda F, Izsvák Z, Ivics Z. Grabundzija I, et al. Nat Commun. 2016 Mar 2;7:10716. doi: 10.1038/ncomms10716. Nat Commun. 2016. PMID: 26931494 Free PMC article. - Design of Novel Relaxase Substrates Based on Rolling Circle Replicases for Bioconjugation to DNA Nanostructures.
Sagredo S, de la Cruz F, Moncalián G. Sagredo S, et al. PLoS One. 2016 Mar 30;11(3):e0152666. doi: 10.1371/journal.pone.0152666. eCollection 2016. PLoS One. 2016. PMID: 27027740 Free PMC article.
References
- Kornberg A & Baker TA DNA Replication 2nd edn (Freeman, 1992).
- Koonin EV & Ilyina TV Computer-assisted dissection of rolling circle DNA replication. Biosystems 30, 241–268 (1993).
References 2 and 3 are the first bioinformatic analyses of HUH endonucleases.
- Koonin EV & Ilyina TV Computer-assisted dissection of rolling circle DNA replication. Biosystems 30, 241–268 (1993).
- Garcillan-Barcia MP, Bernales I, Mendiola MV & De la Cruz F in Mobile DNA Vol. II (eds Craig NL, Craigie R, Gellert M, & Lambowitz A) 891–904 (ASM Press, 2002).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources