The HCMV gH/gL/UL128-131 complex triggers the specific cellular activation required for efficient viral internalization into target monocytes - PubMed (original) (raw)
Comparative Study
The HCMV gH/gL/UL128-131 complex triggers the specific cellular activation required for efficient viral internalization into target monocytes
Maciej T Nogalski et al. PLoS Pathog. 2013.
Abstract
We have established that HCMV acts as a specific ligand engaging and activating cellular integrins on monocytes. As a result, integrin signaling via Src activation leads to the functional activation of paxillin required for efficient viral entry and for the biological changes in monocytes needed for viral dissemination. These biological/molecular changes allow HCMV to use monocytes as "vehicles" for systemic spread and the establishment of lifelong persistence. However, it remains unresolved how HCMV specifically induces this observed monocyte activation. It was previously demonstrated that the HCMV gH/gL/UL128-131 glycoprotein complex facilitates viral entry into biologically relevant cell types. Nevertheless, the mechanism by which the gH/gL/UL128-131 complex promotes this process is unknown. We now show that only HCMV virions possessing the gH/gL/UL128-131 complex are capable of activating integrin/Src/paxillin-signaling in monocytes. In fibroblasts, this signaling is reversed, such that virus lacking the gH/gL/UL128-131 complex is the only virus able to induce the paxillin activation cascade. The presence of the gH/gL/UL128-131 complex also may have an inhibitory effect on integrin-mediated signaling pathway in fibroblasts. Furthermore, we demonstrate that the presence of the gH/gL/UL128-131 complex on the viral envelope, through its activation of the integrin/Src/paxillin pathway, is necessary for efficient HCMV internalization into monocytes and that appropriate actin and dynamin regulation is critical for this entry process. Importantly, productive infection in monocyte-derived macrophages was seen only in cells exposed to HCMV expressing the gH/gL/UL128-131 complex. From our data, the HCMV gH/gL/U128-131 complex emerges as the specific ligand driving the activation of the receptor-mediated signaling required for the regulation of the actin cytoskeleton and, consequently, for efficient and productive internalization of HCMV into monocytes. To our knowledge, our studies demonstrate a possible molecular mechanism for why the gH/gL/UL128-131 complex dictates HCMV tropism and why the complex is lost as clinical isolates are passaged in the laboratory.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
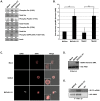
Figure 1. Presence of the gH/gL/UL128-131 complex links HCMV′s ability to activate and efficiently enter into target monocytes.
(A) Approximately 2×106 virions of Towne (p.40, p.51 and p.57), AD169, TB40/E and TB40/F were spun down through a sucrose cushion, lysed and western blot analyses were performed using antibodies recognizing the HCMV proteins, pp65 and pUL130. (B) Monocytes were isolated and cultured in low serum for 24 h at 37°C/5% CO2. Monocytes were then mock- or HCMV (Towne p.40, Towne p.57, TB40/E, TB40/F, AD169)-infected (M.O.I. of 5) and harvested at 15 min. pi. Western blot analyses were performed using antibodies specific for the phosphorylated and non-phosphorylated forms of Src and p70 S6 kinase. Actin was used as a loading control. The results were also measured by densitometry with relative numbers shown in the figure. (C) Monocytes were mock infected or HCMV (Towne p.40, Towne p.57, TB40/E, TB40/F, AD169) infected (M.O.I. of 0.1) for 1 h at 4°C, then temperature shifted to 37°C for 1 h. Monocytes were washed and treated with Proteinase K solution for 1 h. Monocytes were then harvested and semi-quantitative PCR was performed using primers complementary to genomic HCMV DNA and cellular GAPDH, as an internal control. PCR reactions were analyzed by agarose gel electrophoresis using ethidium bromide. (D, E, F, G, H, I and J) Monocytes were mock- or HCMV (TB40/E or AD169)-infected (M.O.I. of 5) for 1 h at 4°C, then 2 mM of DTSSP [3,3′-dithiobis (sulfosuccinimidylpropionate] was added at 4°C for additional 2 h. Cells were spun down and lysed. Antibodies recognizing β1, β3 integrins, HCMV gH or isotype control IgG were added overnight at 4°C to cellular lysates and then protein A/G Sepharose was added for 4 h at 4°C. Protein A/G Sepharose beads with bound protein complexes were spun down, washed with a lysis buffer and resuspended in sample buffer. Western blot analyses were performed using antibodies recognizing β1 and β3 integrins, as well as the HCMV gH and pUL130. Lysates from HCMV-infected monocytes were also analyzed for equal levels of β1, β3 integrins and actin in samples undergoing immunoprecipitation. All experiments were repeated at least three times and representative results are shown. Note: The arrows point to the band of interest. The asterisks mark non-specific bands. (K) The schematic diagram describes our cumulative data from the immunoprecipitation analysis; illustrating the interaction between the gH/gL/UL128-131 complex of TB40/E strain or gH/gL/(gO) complex of AD169 strain with cellular integrins.
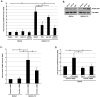
Figure 2. The HCMV gH/gL/UL128-131 complex is critical for activation of, and for efficient and productive viral internalization into target monocytes.
(A) Monocytes were cultured in low serum for 24 h at 37°C/5% CO2. Monocytes were then mock- or HCMV (BAD_wt_ or BAD_r_UL131)-infected (M.O.I. of 5) and harvested at 15 min. pi. Western blot analyses were performed using antibodies specific for the phosphorylated and non-phosphorylated forms of Src, paxillin, Erk and SAPK/JNK. Actin was used as a loading control. The results were also measured by densitometry with relative numbers shown in the figure. (B) Monocytes were HCMV (BADwt, BADrUL131, TB40/F or TB40/E)-infected (M.O.I. of 0.1) for 1 h at 4°C, then temperature shifted to 37°C for 1 h. Monocytes were washed and treated with Proteinase K solution for 1 h. Monocytes were then harvested and quantitative real-time PCR was performed using primers complementary to genomic HCMV DNA and 18S rRNA, as an internal control. Results are plotted as a mean ±SEM. Student's T-tests were performed and p<0.05 (indicated by asterisks) was used for the measurement of statistical significance between samples. (C and D) Monocytes were mock- or HCMV (BAD_wt_ or BAD_r_UL131)-infected [M.O.I. of 5 (C) or 1 (D)] and incubated at 37°C in 5% CO2 for 5 days. (C) Cells were fixed, cytospun, underwent fluorescence in situ hybridization and were analyzed using a confocal microscopy (vDNA – yellow/green & DAPI nuclear stain – pink) or (D) were harvested and semiquantitative PCR was performed using primers complementary to genomic HCMV DNA and 18S rRNA, as an internal control. Note: in the (C) panel, inset pictures are an enlarged version of the representative cell shown in the picture. (E) Monocytes were HCMV (BAD_wt_ or BAD_r_UL131)-infected (M.O.I. of 1) and incubated at 37°C in 5% CO2 for 3 weeks. RNA was harvested from cells, reverse transcribed and semiquantitative PCR was performed using primers complementary to HCMV IE1-72 mRNA and 18S rRNA, as an internal control. Reverse transcriptase negative (RT-) sample was also tested to demonstrate lack of residual, genomic DNA in samples. All experiments were repeated at least three times and representative results are shown.
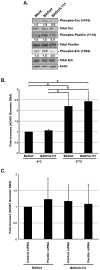
Figure 3. Integrin engagement and activation of the integrin/Src/paxillin signaling pathway by the gH/gL/UL128-131 complex allows efficient HCMV internalization into target monocytes.
(A and B) Monocytes were pretreated with 1 µM PP2, 1 µM AG1478, 5 µg/ml of blocking anti-β1 or anti-β3 integrin antibodies, or 5 µg/ml of IgG for 1 h at 37°C/5% CO2. (C and D) Monocytes were transfected with siRNA complementary to paxillin or a control siRNA for 48 h. (A, B, C, and D) Monocytes were then HCMV (BADwt or BADrUL131)-infected (M.O.I. of 0.1) for 1 h at 4°C, washed, treated with 5 U/ml of α-thrombin (D only), then temperature shifted to 37°C for 1 h. Monocytes were washed and treated with Proteinase K solution for 1 h. Monocytes were then harvested and quantitative real-time PCR was performed using primers complementary to genomic HCMV DNA and 18S rRNA, as an internal control. Results are plotted as a mean ±SEM. Student's T-tests were performed and p<0.05 (indicated by asterisks) was used for the measurement of statistical significance between samples. All experiments were repeated at least three times.
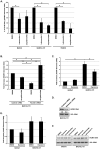
Figure 4. Presence of the gH/gL/UL128-131 complex has no effect on the integrin/Src/paxillin signaling pathway in and HCMV internalization into fibroblasts.
(A) Fibroblasts were cultured in low serum for 24 h at 37°C/5% CO2. Fibroblasts were then mock- or HCMV (BADwt or BADrUL131)-infected (M.O.I. of 5) and harvested at 20 min. pi. Western blot analyses were performed using antibodies specific for the phosphorylated and non-phosphorylated forms of Src, paxillin, and Erk. Actin was used as a loading control. The results were also measured by densitometry with relative numbers shown in the figure. (B) Fibroblasts were infected with BADwt or BADrUL131 (M.O.I. of 0.1) for 1 h at 4°C, then left at 4°C or temperature shifted to 37°C for 1 h. (C) Fibroblasts were transfected with siRNA complementary to paxillin or a control siRNA for 48 h. Fibroblasts were infected with BADwt or BADrUL131 (M.O.I. of 0.1) for 1 h at 4°C, then temperature shifted to 37°C for 1 h. (B and C) Then, fibroblasts were washed and treated with Proteinase K solution for 1 h. Cells were then harvested and quantitative real-time PCR was performed using primers complementary to genomic HCMV DNA and 18S rRNA, as an internal control. Results are plotted as a mean ±SEM. Student's T-tests were performed and p<0.05 (indicated by asterisks) was used for the measurement of statistical significance between samples. All experiments were repeated at least three times and representative results are shown.
Figure 5. Regulation of the actin cytoskeleton and dynamin is essential for efficient internalization of clinical-like HCMV isolates into monocytes, but not into fibroblasts.
(A) Monocytes were pretreated with DMSO, 0.5 µM jasplakinolide, or 2.5 µM latrunculin A. (B) Monocytes were transfected with siRNA complementary to paxillin or a control siRNA for 48 h. (C) Monocytes were preatreated with 50 µM dynasore. (D) Monocytes were transfected with siRNA complementary to dynamin or a control siRNA for 48 h. (E and F) Fibroblasts were pretreated with DMSO, 0.5 µM jasplakinolide, 2.5 µM latrunculin A, or 50 µM dynasore. (A, B, C, D, E, and F) Cells were then infected with BADwt, BADrUL131 or TB40/E (M.O.I. of 0.1) for 1 h at 4°C washed and then temperature shifted to 37°C for 1 h. Monocytes were then treated with 0.5 µM jasplakinolide at 37°C for an additional 1 h (only B). Cells were then washed and treated with Proteinase K solution for 1 h. Cells were harvested and PCR was performed using primers complementary to genomic HCMV DNA and 18S rRNA. For qPCR data, results are plotted as a mean ±SEM. Student's T-tests were performed and p<0.05 (indicated by asterisks) was used for the measurement of statistical significance between samples. For semiquantitative PCR, PCR reactions were analyzed by agarose gel electrophoresis using ethidium bromide. The experiments were performed at least three times.
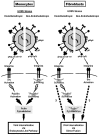
Figure 6. Our model: The HCMV gH/gL/UL128-131 complex differs in its ability to trigger functional cellular activation in monocytes vs. fibroblasts, causing contrasting modes of entry into these cell types.
On monocytes, the gH/gL/UL128-131 and gH/gL/(gO) complexes engage both β1 and β3 integrins, however, only the pentameric complex triggers integrin-mediated signaling. In the case of the gH/gL/UL128-131 complex, the gH protein interacts with β1 integrins, and the UL128-131 complex engages both β1 and β3 integrins, suggesting that the UL128-131 complex allows for cooperative regulation of receptor-mediated signaling pathway(s) initiated through the activation of separate integrin heterodimers to create the appropriate type/level of receptor-mediated signaling beneficial to the virus. This signal transduction process is consequently responsible for the functional activation/phosphorylation of paxillin (lightning bolts), which governs the rearrangement of the actin cytoskeleton ultimately leading to efficient internalization of HCMV into monocytes via an endocytosis-like pathway (black arrows). On fibroblasts, the gH/gL/(gO) complex(es) triggers integrin/Src-mediated signaling, however, paxillin-dependent actin rearrangement is not important for HCMV internalization, suggesting an involvement of contrasting molecular pathways in HCMV-infected fibroblasts (dotted arrows) vs. those seen in monocytes, leading to different modes of HCMV entry into fibroblasts vs. monocytes.
Similar articles
- Human Cytomegalovirus gH/gL/gO Promotes the Fusion Step of Entry into All Cell Types, whereas gH/gL/UL128-131 Broadens Virus Tropism through a Distinct Mechanism.
Zhou M, Lanchy JM, Ryckman BJ. Zhou M, et al. J Virol. 2015 Sep;89(17):8999-9009. doi: 10.1128/JVI.01325-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085146 Free PMC article. - Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions.
Ryckman BJ, Chase MC, Johnson DC. Ryckman BJ, et al. J Virol. 2010 Mar;84(5):2597-609. doi: 10.1128/JVI.02256-09. Epub 2009 Dec 23. J Virol. 2010. PMID: 20032193 Free PMC article. - A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells.
Wille PT, Knoche AJ, Nelson JA, Jarvis MA, Johnson DC. Wille PT, et al. J Virol. 2010 Mar;84(5):2585-96. doi: 10.1128/JVI.02249-09. Epub 2009 Dec 23. J Virol. 2010. PMID: 20032184 Free PMC article. - Characteristics and functions of human cytomegalovirus UL128 gene/protein.
Tao R, Xu J, Gao H-, Zhao W-, Shang S-. Tao R, et al. Acta Virol. 2014;58(2):103-7. doi: 10.4149/av_2014_02_103. Acta Virol. 2014. PMID: 24957713 Review. - Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.
Nguyen CC, Kamil JP. Nguyen CC, et al. Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
Cited by
- The Pentamer glycoprotein complex inhibits viral Immediate Early transcription during Human Cytomegalovirus infections.
Ohman MS, Albright ER, Gelbmann CB, Kalejta RF. Ohman MS, et al. Proc Natl Acad Sci U S A. 2024 Sep 24;121(39):e2408078121. doi: 10.1073/pnas.2408078121. Epub 2024 Sep 18. Proc Natl Acad Sci U S A. 2024. PMID: 39292744 - Distinct early role of PTEN regulation during HCMV infection of monocytes.
Chesnokova LS, Mosher BS, Fulkerson HL, Nam HW, Shakya AK, Yurochko AD. Chesnokova LS, et al. Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2312290121. doi: 10.1073/pnas.2312290121. Epub 2024 Mar 14. Proc Natl Acad Sci U S A. 2024. PMID: 38483999 Free PMC article. - Human cytomegalovirus modulates mTORC1 to redirect mRNA translation within quiescently infected monocytes.
Miller MJ, Akter D, Mahmud J, Chan GC. Miller MJ, et al. J Virol. 2024 Feb 20;98(2):e0188823. doi: 10.1128/jvi.01888-23. Epub 2024 Jan 30. J Virol. 2024. PMID: 38289104 Free PMC article. - Insights into the Transcriptome of Human Cytomegalovirus: A Comprehensive Review.
Zeng J, Cao D, Yang S, Jaijyan DK, Liu X, Wu S, Cruz-Cosme R, Tang Q, Zhu H. Zeng J, et al. Viruses. 2023 Aug 8;15(8):1703. doi: 10.3390/v15081703. Viruses. 2023. PMID: 37632045 Free PMC article. Review. - Human cytomegalovirus in cancer: the mechanism of HCMV-induced carcinogenesis and its therapeutic potential.
Yu C, He S, Zhu W, Ru P, Ge X, Govindasamy K. Yu C, et al. Front Cell Infect Microbiol. 2023 Jun 23;13:1202138. doi: 10.3389/fcimb.2023.1202138. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37424781 Free PMC article. Review.
References
- Speir E, Modali R, Huang ES, Leon MB, Shawl F, et al. (1994) Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265: 391–394. - PubMed
- Melnick JL, Adam E, DeBakey ME (1995) Cytomegalovirus and atherosclerosis. Bioessays 17: 899–903. - PubMed
- Streblow DN, Vomaske J, Smith P, Melnychuk R, Hall L, et al. (2003) Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. J Biol Chem 278: 50456–50465. - PubMed
- Waldman WJ, Adams PW, Knight DA, Sedmak DD (1997) CMV as an exacerbating agent in transplant vascular sclerosis: potential immune-mediated mechanisms modelled in vitro. Transplant Proc 29: 1545–1546. - PubMed
- Streblow DN, Orloff SL, Nelson JA (2001) Do pathogens accelerate atherosclerosis? J Nutr 131: 2798S–2804S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 GM110703/GM/NIGMS NIH HHS/United States
- R01 AI056077/AI/NIAID NIH HHS/United States
- R01 HD051998/HD/NICHD NIH HHS/United States
- P20-RR018724/RR/NCRR NIH HHS/United States
- P20 GM103433/GM/NIGMS NIH HHS/United States
- HD-051998/HD/NICHD NIH HHS/United States
- AI050677/AI/NIAID NIH HHS/United States
- P20 RR018724/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous