Leukemia-associated RhoGEF (LARG) is a novel RhoGEF in cytokinesis and required for the proper completion of abscission - PubMed (original) (raw)
Leukemia-associated RhoGEF (LARG) is a novel RhoGEF in cytokinesis and required for the proper completion of abscission
Matthew K Martz et al. Mol Biol Cell. 2013 Sep.
Abstract
Proper completion of mitosis requires the concerted effort of multiple RhoGEFs. Here we show that leukemia-associated RhoGEF (LARG), a RhoA-specific RGS-RhoGEF, is required for abscission, the final stage of cytokinesis, in which the intercellular membrane is cleaved between daughter cells. LARG colocalizes with α-tubulin at the spindle poles before localizing to the central spindle. During cytokinesis, LARG is condensed in the midbody, where it colocalizes with RhoA. HeLa cells depleted of LARG display apoptosis during cytokinesis with unresolved intercellular bridges, and rescue experiments show that expression of small interfering RNA-resistant LARG prevents this apoptosis. Moreover, live cell imaging of LARG-depleted cells reveals greatly delayed fission kinetics in abscission in which a population of cells with persistent bridges undergoes apoptosis; however, the delayed fission kinetics is rescued by Aurora-B inhibition. The formation of a Flemming body and thinning of microtubules in the intercellular bridge of cells depleted of LARG is consistent with a defect in late cytokinesis, just before the abscission event. In contrast to studies of other RhoGEFs, particularly Ect2 and GEF-H1, LARG depletion does not result in cytokinetic furrow regression nor does it affect internal mitotic timing. These results show that LARG is a novel and temporally distinct RhoGEF required for completion of abscission.
Figures
FIGURE 1:
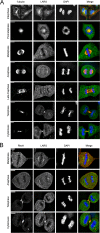
Mitotic distribution of LARG. (A, B) HeLa cells were fixed, and α-tubulin (red), RhoA (red), LARG (green; SC H70), and DNA (blue) were visualized by immunofluorescence microscopy, as described in Materials and Methods. Bar, 11 μm.
FIGURE 2:
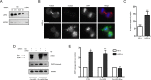
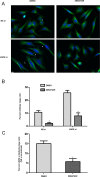
Depletion of LARG results in apoptosis. **(**A) HeLa cells were treated with non- NS or LARG siRNA as described in Materials and Methods. Efficiency of endogenous LARG depletion (>90%) is shown by an immunoblot of cell lysates comparing the LARG signal in LARG siRNA lysates to decreasing equivalents of NS cell lysates. (B) HeLa cells were treated with LARG siRNA and fixed 12 h after release from double-thymidine G1/S synchronization to enrich for cells undergoing mitosis. Cells were stained for α-tubulin (red), cleaved caspase-3 (green), and DNA (blue). Images show examples of normal (top) and apoptotic (bottom) cytokinesis in LARG siRNA–treated cells. Bar, 10 μm. (C) Quantification of the percentage of cleaved caspase-3–positive cells (**p < 0.05, n = 1438 NS si cells, 1021 LARG si cells). (D) HeLa cells were transfected with the indicated siRNA together with expression plasmids for mCherry-LARG (Ch-LARG) or the siRNA-resistant Ch-LARG-5Res and analyzed by immunoblot for PARP-1 cleavage as described in Materials and Methods. The arrows indicate the position of endogenous LARG (endo) and exogenous mCherry-tagged LARG (exo). (E) Quantification of PARP-1 cleavage as fold change over control siRNA–treated cells normalized for HSP90 loading control (*p = 0.0008, **p < 0.02, n = 3 independent experiments).
FIGURE 3:
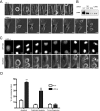
Depletion of LARG results in apoptosis in late cytokinesis. HeLa cells stably expressing GFP-H2B were treated with the indicated siRNA and synchronized at G1/S by the double-thymidine method. Cells were released from G1/S block and imaged by live cell microscopy with times indicated in minutes. (A) Bright-field images of cells progressing through mitosis after treatment with indicated siRNAs. Arrows highlight intercellular bridge in LARG si–treated cells. (B) Representative Western blot of the depletion of LARG in imaged cells. (C) GFP-H2B and bright-field images of LARG si–treated cells showing the classic degradation of DNA and membrane blebbing indicative of apoptosis after unresolved cytokinesis. Arrows highlight intercellular bridges. (D) Quantification of fates of NS and LARG si–treated cells that enter mitosis. Values expressed as a percentage of total number of cells entering mitosis during filming (**p < 0.0005, n = 262 NS si cells, 297 LARG si cells across three independent experiments).
FIGURE 4:
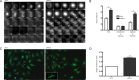
Cells depleted of LARG undergo normal mitotic kinetics but are unable to complete abscission. (A) HeLa cells stably expressing GFP–α-tubulin were treated with the indicated siRNA, synchronized at G1/S by the double-thymidine method, and imaged by live cell microscopy beginning just before initiation of mitosis (times indicated in minutes). Arrow highlights presence of α-tubulin in LARG si cells well after control NS si cells have completed abscission. (B) Quantification of mitotic kinetics as measured by α-tubulin dynamics (*p < 0.0001, n = 97 NS si cells, n = 85 LARG si cells). Total, initiation of mitosis to intercellular α-tubulin resolution; condensation to ingression, initiation of mitosis to completion of contractile ring ingression; ingression to resolution, completion of contractile ring ingression to intercellular α-tubulin resolution. (C) HeLa cells were treated with siRNA, fixed 12 h after release from double thymidine, and stained for α-tubulin (green) and DNA (blue). Arrows highlight intercellular bridges that have completed contractile ring ingression. LARG si inset highlights Flemming body with proper midbody matrix (red arrow) and distal thinning of microtubules in the intercellular bridge (flanking white arrows). Bar, 10 μm. (D) Quantification of cells connected by persistent α-tubulin–containing intercellular bridges as fold over control NS si cells (**p < 0.0005, n ≥1500 cells for each siRNA across three independent experiments).
FIGURE 5:
Cells depleted of LARG undergo an Aurora-B–dependent delay in abscission kinetics. (A) Asynchronous HeLa cells were treated with siRNA for 72 h. Cells were treated with either DMSO vehicle or 2 μM ZM447439 to inhibit Aurora-B for 2 h before fixing and staining for α-tubulin (green) and DNA (blue). Arrows highlight midbody-stage intercellular bridges. Bar, 10 μm. (B) Quantification of the percentage of midbody-stage intercellular bridges for both NS si and LARG si cells in the presence of vehicle or Aurora-B inhibitor (*p ≤ 0.0012, DMSO vs. ZM447439; DMSO, NS si = 7478 cells, LARG si = 3388 cells; ZM447439, NS si = 7229 cells, LARG si = 4249 cells; across four independent experiments). (C) Percentage of midbody-stage intercellular bridges for LARG si cells normalized by subtraction of NS si cell percentages for each DMSO and ZM447439 treatment (*p = 0.0045).
FIGURE 6:
Y940 of the DH core of LARG is critical for binding to RhoA, and mutation leads to apoptosis. (A) HeLa cells were transfected with indicated plasmid. At 48 h posttransfection, cell lysates were harvested and subject to GST-G17A-RhoA pull down. Relative binding to RhoA was determined by immunoblot for LARG and pull down and input fractions compared for each respective LARG plasmid. I, 5% total input; P, 50% total pull down. Top bands, exogenous mCherry-tagged LARG proteins; bottom bands, endogenous LARG. (B) HeLa cells were transfected with indicated plasmid for 48 h, cell lysates harvested, and relative apoptosis determined by immunoblot for PARP cleavage. (C) Crystal structure of DH/PH domain of LARG in complex with RhoA from PDB database (1X86; Kristelly et al., 2003) depicting residues (pink) of the DH domain (gray) targeted here in mutation studies and relative locations to RhoA (brown). (D) Multiple RhoGEFs exhibit temporally distinct functions for the proper completion of mitosis. See the text for discussion.
Similar articles
- Mitotic-dependent phosphorylation of leukemia-associated RhoGEF (LARG) by Cdk1.
Helms MC, Grabocka E, Martz MK, Fischer CC, Suzuki N, Wedegaertner PB. Helms MC, et al. Cell Signal. 2016 Jan;28(1):43-52. doi: 10.1016/j.cellsig.2015.10.004. Epub 2015 Oct 19. Cell Signal. 2016. PMID: 26483157 Free PMC article. - Leukemia-associated Rho guanine nucleotide exchange factor promotes G alpha q-coupled activation of RhoA.
Booden MA, Siderovski DP, Der CJ. Booden MA, et al. Mol Cell Biol. 2002 Jun;22(12):4053-61. doi: 10.1128/MCB.22.12.4053-4061.2002. Mol Cell Biol. 2002. PMID: 12024019 Free PMC article. - The leukemia-associated Rho guanine nucleotide exchange factor LARG is required for efficient replication stress signaling.
Beveridge RD, Staples CJ, Patil AA, Myers KN, Maslen S, Skehel JM, Boulton SJ, Collis SJ. Beveridge RD, et al. Cell Cycle. 2014;13(21):3450-9. doi: 10.4161/15384101.2014.956529. Cell Cycle. 2014. PMID: 25485589 Free PMC article. - Spatiotemporal Regulation of RhoA during Cytokinesis.
Basant A, Glotzer M. Basant A, et al. Curr Biol. 2018 May 7;28(9):R570-R580. doi: 10.1016/j.cub.2018.03.045. Curr Biol. 2018. PMID: 29738735 Free PMC article. Review. - The Abscission Checkpoint: A Guardian of Chromosomal Stability.
Petsalaki E, Zachos G. Petsalaki E, et al. Cells. 2021 Nov 29;10(12):3350. doi: 10.3390/cells10123350. Cells. 2021. PMID: 34943860 Free PMC article. Review.
Cited by
- Cytokinetic abscission is an acute G1 event.
Gershony O, Pe'er T, Noach-Hirsh M, Elia N, Tzur A. Gershony O, et al. Cell Cycle. 2014;13(21):3436-41. doi: 10.4161/15384101.2014.956486. Cell Cycle. 2014. PMID: 25485587 Free PMC article. - SRRF-Stream Imaging of Optogenetically Controlled Furrow Formation Shows Localized and Coordinated Endocytosis and Exocytosis Mediating Membrane Remodeling.
Castillo-Badillo JA, Bandi AC, Harlalka S, Gautam N. Castillo-Badillo JA, et al. ACS Synth Biol. 2020 Apr 17;9(4):902-919. doi: 10.1021/acssynbio.9b00521. Epub 2020 Mar 16. ACS Synth Biol. 2020. PMID: 32155337 Free PMC article. - Screening and identification of critical transcription factors involved in the protection of cardiomyocytes against hydrogen peroxide-induced damage by Yixin-shu.
Zhang J, Geng Y, Guo F, Zhang F, Liu M, Song L, Ma Y, Li D, Zhang Y, Xu H, Yang H. Zhang J, et al. Sci Rep. 2017 Oct 24;7(1):13867. doi: 10.1038/s41598-017-10131-5. Sci Rep. 2017. PMID: 29066842 Free PMC article. - Therapy-induced Deletion in 11q23 Leading to Fusion of KMT2A With ARHGEF12 and Development of B Lineage Acute Lymphoplastic Leukemia in a Child Treated for Acute Myeloid Leukemia Caused by t(9;11)(p21;q23)/KMT2A-MLLT3.
Panagopoulos I, Andersen K, Eilert-Olsen M, Zeller B, Munthe-Kaas MC, Buechner J, Osnes LTN, Micci F, Heim S. Panagopoulos I, et al. Cancer Genomics Proteomics. 2021 Jan-Feb;18(1):67-81. doi: 10.21873/cgp.20242. Epub 2021 Jan 8. Cancer Genomics Proteomics. 2021. PMID: 33419897 Free PMC article. - Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells.
Chircop M. Chircop M. Small GTPases. 2014;5:e29770. doi: 10.4161/sgtp.29770. Epub 2014 Jul 2. Small GTPases. 2014. PMID: 24988197 Free PMC article. Review.
References
- Becknell B, Shen T, Maghraby E, Taya S, Kaibuchi K, Caligiuri MA, Marcucci G. Characterization of leukemia-associated Rho guanine nucleotide exchange factor (LARG) expression during murine development. Cell Tissue Res. 2003;314:361–366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous