The role of AKT/mTOR pathway in stress response to UV-irradiation: implication in skin carcinogenesis by regulation of apoptosis, autophagy and senescence - PubMed (original) (raw)
Review
The role of AKT/mTOR pathway in stress response to UV-irradiation: implication in skin carcinogenesis by regulation of apoptosis, autophagy and senescence
Elwira Strozyk et al. Int J Mol Sci. 2013.
Abstract
Induction of DNA damage by UVB and UVA radiation may generate mutations and genomic instability leading to carcinogenesis. Therefore, skin cells being repeatedly exposed to ultraviolet (UV) light have acquired multilayered protective mechanisms to avoid malignant transformation. Besides extensive DNA repair mechanisms, the damaged skin cells can be eliminated by induction of apoptosis, which is mediated through the action of tumor suppressor p53. In order to prevent the excessive loss of skin cells and to maintain the skin barrier function, apoptotic pathways are counteracted by anti-apoptotic signaling including the AKT/mTOR pathway. However, AKT/mTOR not only prevents cell death, but is also active in cell cycle transition and hyper-proliferation, thereby also counteracting p53. In turn, AKT/mTOR is tuned down by the negative regulators being controlled by the p53. This inhibition of AKT/mTOR, in combination with transactivation of damage-regulated autophagy modulators, guides the p53-mediated elimination of damaged cellular components by autophagic clearance. Alternatively, p53 irreversibly blocks cell cycle progression to prevent AKT/mTOR-driven proliferation, thereby inducing premature senescence. Conclusively, AKT/mTOR via an extensive cross talk with p53 influences the UV response in the skin with no black and white scenario deciding over death or survival.
Figures
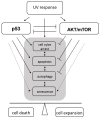
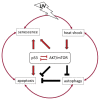
Figure 1
The role of p53 and AKT/mTOR in cellular responses to ultraviolet (UV) radiation. UV activates both, p53 and AKT/mTOR signaling pathways. An intact p53 response in irradiated cells leads to cell cycle arrest to enable damage repair and eventually to induce apoptotic cell death when the damage is too severe and/or repair remains incomplete. Cell cycle arrest and apoptosis are negatively regulated by AKT/mTOR activity. Thus, AKT/mTOR can enforce proliferation. It also prevents autophagy, a mechanism to recycle damaged proteins or organelles that remain under the control of p53. So far, AKT/mTOR can counteract the activity of p53 in response to UV irradiation and vice versa. At last, p53 in concert with AKT/mTOR signaling can drive cells to premature senescence, an irreversible cell-cycle arrest that counteracts oncogenic transformation. Shifting the balance between p53 and AKT/mTOR signaling can determine between either cell death or survival and clonal expansion of irradiated cells.
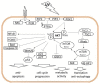
Figure 2
Oncogenic role of the AKT signaling pathway. UV-triggered RTK or functional inactivation of PTEN leads to activation of AKT. Activated AKT signals to activate the anti-apoptotic transcription factor NFκB or inhibits pro-apoptotic molecules such as the transcription factor FOXO, Bad, or caspase 9. By activation of the p53 inhibitor MDM2, AKT antagonizes p53-mediated responses: i.e., cell cycle arrest and apoptosis induction. AKT forces cell cycle progression by blocking cell cycle control proteins p21 and p27, and via inhibition of GSK3 kinase stabilizes cyclins and drives cellular metabolism. AKT-mediated inhibition of TSC2 leads to activation of mTOR/Raptor (mTORC1), which controls protein synthesis and autophagy. Activated by a currently unknown mechanism mTOR/Rictor (mTORC2) mediates critical phosphorylation and activation of AKT.
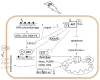
Figure 3
p53-induced cell cycle control and apoptosis. UV-induced DNA damage activates ATR, ATM and DNA-PK kinases, which via check point kinases Chk1/2 signal to activate p53 and DNA damage repair. Activated p53 transcriptionally regulates the cell cycle control protein p21 and several components of the pro-apoptotic pathway. Pro-apoptotic Bax, Bak, Noxa and PUMA proteins and additionally death receptors CD95 and DR5 become up-regulated while p53 trans-represses anti-apoptotic Bcl-2 and Bcl-xL. Moreover, p53 induces apoptosis by direct interaction with the mitochondrial membrane. UV-activated AKT inhibits p53 by activation of its regulator MDM2 and/or by inhibition of Chk1/2. Above that, by inhibition of Chk1/2 AKT may interfere with DNA damage repair and directly inhibit p21.
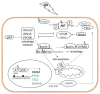
Figure 4
The interplay between p53 and AKT/mTOR in regulating autophagy. UV stress-induced p53 regulates the expression of damage-regulated autophagy modulator (DRAM) and factors mediating inhibition of AKT/mTOR (in blue). Inhibition of mTOR results in activation of ULK1/2–ATG13–Fip200 autophagy initiating complex. Consequently, dissociation of Beclin-1/Bcl complex enables Beclin-1 to interact with UVRAG activating next steps in the process of autophagy. Bcl proteins can now exert their anti-apoptotic function by counteracting Bak or Bax. The pro-apoptotic function of Bax can additionally be inhibited by up-regulated UVRAG.
Figure 5
The balance between p53 and AKT/mTOR determines the fate of the UV-irradiated cells. p53 and AKT/mTOR mutually inhibit each other in a tightly regulated cross talk, that influences cellular responses at different levels. UV-induced p53-dependent apoptosis can be inhibited by UV- or heat-activated AKT/mTOR pathway. Vice versa, UV-activated p53 can inhibit AKT/mTOR thereby counteracting its anti-apoptotic function but contributing to induction of autophagy. However, UV-induced autophagy counteracts UV-induced apoptosis at least to certain extends. Under slightly different physiological conditions, concomitant activation of p53 and AKT/mTOR can drive cells into senescence and thereby mediate an anti-tumor response. Thus, a very complex regulation of cell death and survival pathways exists, that controls malignant transformation of skin cells.
Similar articles
- Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes: A new implication of skin cancer prevention.
Bridgeman BB, Wang P, Ye B, Pelling JC, Volpert OV, Tong X. Bridgeman BB, et al. Cell Signal. 2016 May;28(5):460-468. doi: 10.1016/j.cellsig.2016.02.008. Epub 2016 Feb 12. Cell Signal. 2016. PMID: 26876613 Free PMC article. - Cancer cell survival following DNA damage-mediated premature senescence is regulated by mammalian target of rapamycin (mTOR)-dependent Inhibition of sirtuin 1.
Back JH, Rezvani HR, Zhu Y, Guyonnet-Duperat V, Athar M, Ratner D, Kim AL. Back JH, et al. J Biol Chem. 2011 May 27;286(21):19100-8. doi: 10.1074/jbc.M111.240598. Epub 2011 Apr 6. J Biol Chem. 2011. PMID: 21471201 Free PMC article. - Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway.
Lin CJ, Chen TL, Tseng YY, Wu GJ, Hsieh MH, Lin YW, Chen RM. Lin CJ, et al. Toxicol Appl Pharmacol. 2016 Aug 1;304:59-69. doi: 10.1016/j.taap.2016.05.018. Epub 2016 May 25. Toxicol Appl Pharmacol. 2016. PMID: 27236003 - p53 regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment.
Feng Z. Feng Z. Cold Spring Harb Perspect Biol. 2010 Feb;2(2):a001057. doi: 10.1101/cshperspect.a001057. Cold Spring Harb Perspect Biol. 2010. PMID: 20182617 Free PMC article. Review. - DNA damage responses in skin biology--implications in tumor prevention and aging acceleration.
Nakanishi M, Niida H, Murakami H, Shimada M. Nakanishi M, et al. J Dermatol Sci. 2009 Nov;56(2):76-81. doi: 10.1016/j.jdermsci.2009.09.001. Epub 2009 Sep 24. J Dermatol Sci. 2009. PMID: 19781914 Review.
Cited by
- Environmental pollutants and phosphoinositide signaling in autoimmunity.
Ren C, Carrillo ND, Cryns VL, Anderson RA, Chen M. Ren C, et al. J Hazard Mater. 2024 Mar 5;465:133080. doi: 10.1016/j.jhazmat.2023.133080. Epub 2023 Dec 1. J Hazard Mater. 2024. PMID: 38091799 Review. - Aloperine executes antitumor effects through the induction of apoptosis and cell cycle arrest in prostate cancer in vitro and in vivo.
Ling Z, Guan H, You Z, Wang C, Hu L, Zhang L, Wang Y, Chen S, Xu B, Chen M. Ling Z, et al. Onco Targets Ther. 2018 May 11;11:2735-2743. doi: 10.2147/OTT.S165262. eCollection 2018. Onco Targets Ther. 2018. PMID: 29785122 Free PMC article. - Identification of Salvia haenkei as gerosuppressant agent by using an integrated senescence-screening assay.
Matic I, Revandkar A, Chen J, Bisio A, Dall'Acqua S, Cocetta V, Brun P, Mancino G, Milanese M, Mattei M, Montopoli M, Alimonti A. Matic I, et al. Aging (Albany NY). 2016 Dec 1;8(12):3223-3240. doi: 10.18632/aging.101076. Aging (Albany NY). 2016. PMID: 27922821 Free PMC article. - Protein activation mapping of human sun-protected epidermis after an acute dose of erythemic solar simulated light.
Einspahr JG, Curiel-Lewandrowski C, Calvert VS, Stratton SP, Alberts DS, Warneke J, Hu C, Saboda K, Wagener EL, Dickinson S, Dong Z, Bode AM, PetricoinIII EF. Einspahr JG, et al. NPJ Precis Oncol. 2017;1:34. doi: 10.1038/s41698-017-0037-7. Epub 2017 Sep 21. NPJ Precis Oncol. 2017. PMID: 29167824 Free PMC article. - Inhibitors of Nucleotide Excision Repair Decrease UVB-Induced Mutagenesis-An In Vitro Study.
Fidrus E, Hegedűs C, Janka EA, Paragh G, Emri G, Remenyik É. Fidrus E, et al. Int J Mol Sci. 2021 Feb 6;22(4):1638. doi: 10.3390/ijms22041638. Int J Mol Sci. 2021. PMID: 33562002 Free PMC article.
References
- National Toxicology Program. U.S. Department of Health and Human Services. Public Health Service. Report on carcinogens. 12th edition. [(accessed on 18 March 2013)]. Available online: http://ntp.niehs.nih.gov/ntp/roc/twelfth/roc12.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous