Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma - PubMed (original) (raw)
. 2013 Aug 26;210(9):1695-710.
doi: 10.1084/jem.20130579. Epub 2013 Jul 29.
Fubin Li, Welby Montalvo-Ortiz, Manuel A Sepulveda, Katharina Bergerhoff, Frederick Arce, Claire Roddie, Jake Y Henry, Hideo Yagita, Jedd D Wolchok, Karl S Peggs, Jeffrey V Ravetch, James P Allison, Sergio A Quezada
Affiliations
- PMID: 23897981
- PMCID: PMC3754863
- DOI: 10.1084/jem.20130579
Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma
Tyler R Simpson et al. J Exp Med. 2013.
Abstract
Treatment with monoclonal antibody specific for cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), an inhibitory receptor expressed by T lymphocytes, has emerged as an effective therapy for the treatment of metastatic melanoma. Although subject to debate, current models favor a mechanism of activity involving blockade of the inhibitory activity of CTLA-4 on both effector (T eff) and regulatory (T reg) T cells, resulting in enhanced antitumor effector T cell activity capable of inducing tumor regression. We demonstrate, however, that the activity of anti-CTLA-4 antibody on the T reg cell compartment is mediated via selective depletion of T reg cells within tumor lesions. Importantly, T reg cell depletion is dependent on the presence of Fcγ receptor-expressing macrophages within the tumor microenvironment, indicating that T reg cells are depleted in trans in a context-dependent manner. Our results reveal further mechanistic insight into the activity of anti-CTLA-4-based cancer immunotherapy, and illustrate the importance of specific features of the local tumor environment on the final outcome of antibody-based immunomodulatory therapies.
Figures
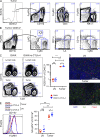
Figure 1.
GVAX+α–CTLA-4 combination therapy protects against tumor outgrowth through a CD4+ T cell–dependent mechanism. (A) Mice were challenged with 104 B16-BL6 on day 0, and then left untreated or treated with GVAX, α–CTLA-4, or GVAX+α–CTLA-4 on day 3, 6, and 8. Results depict tumor growth curves of individual mice (A) and cumulative survival (B). n = 10–25 mice per group, death events correspond to a tumor volume >350 mm3 or death of the mouse. (C) Mice were challenged with 5 × 104 B16-BL6 on day 0 and were treated as described above. The CD4+Foxp3−/CD4+Foxp3+ ratio in the lymph node and tumor was calculated. n = 8–10 mice per group. **, P < 0.01; ***, P < 0.001. Experiments were repeated three times and pooled.
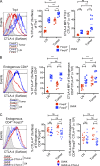
Figure 2.
α–CTLA-4 increases the number of tumor-specific CD4+ T eff and T reg cells in lymph nodes while preventing intratumoral T reg cell accumulation. Mice were challenged with 1.5 × 105 B16-BL6 on day 0, adoptively transferred with 5 × 104 CD4+CD45.1+ Trp1 T on day 3, and treated with GVAX or GVAX+α–CTLA-4 on day 3, 6, and 8. (A) Foxp3 expression by CD4+Vβ14+ cell isolated from Trp1 SJL RAG tan mice before transfer. (B and C) Absolute cell numbers and Foxp3 expression by CD4+CD45.1+ Trp1 T cells from the lymph node (B) and tumor (C). (D) Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 T cells. Mice were treated as described above, but were also left untreated or treated with α–CTLA-4 monotherapy. n = 5 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were repeated two times.
Figure 3.
Multiple α–CTLA-4 clones reduce the intratumoral Trp1 T reg cell population. Mice were treated as described in Fig. 2 with α–CTLA-4 clone 4F10, 9D9, or 9H10. Results depict absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 T cells. n = 5 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiment was repeated two times.
Figure 4.
Tumor-specific T reg cells are rapidly and cell autonomously eliminated from the tumor by α–CTLA-4. (A) Mice were challenged with 1.5 × 105 B16-BL6 on day 0, adoptively transferred with 5 × 104 CD4+CD45.1+ Trp1 T on day 3, and treated with GVAX or GVAX+α–CTLA-4 on day 3, 6, and 8. Some mice received no further treatment or were treated with α–CTLA-4 on day 3–10, or on day 10 alone, and then sacrificed on day 11. Results depict absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 T cells. n = 9–10 mice per group. (B and C) Wild-type and CTLA-4-huTg mice were treated as described in Fig. 2. Results depict absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 (B) and CD4+CD45.1− endogenous polyclonal T cells (C). (D) CTLA-4+/+ and CTLA-4−/− Trp1 T cells were transferred into wild-type mice, and then treated as described in Fig. 2. Results depict absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 T cells. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were repeated two times and pooled.
Figure 5.
Tumor-specific T reg cells are depleted from the tumor by α–CTLA-4. (A) CD4+GFP− cells were FACS purified from CD45.1+/+ BW RAG−/− Trp1 Foxp3-GFP mice, adoptively transferred into mice bearing 3-d established tumors and treated as described in Fig. 2. Results depict Foxp3 expression by CD4+CD45.1+ Trp1 cells in the tumor. (B) Mice were challenged with 1.5 × 105 B16-BL6 on day 0, a mixture of CD25− CD45.1+/+ and CD25+ CD45.1+/− CD45.2+/− Trp1 T cells was adoptively transferred on day 3, and mice were then treated as described in Fig. 2. (top) Phenotype of sorted CD25− and CD25+ Trp1 T cells before mixing and transfer. (bottom) Absolute cell numbers and CD45.2 expression by intratumoral CD4+CD45.1+ Trp1 T cells. n = 5 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were repeated two times.
Figure 6.
Tumor-specific T reg cells are not depleted by complement, the FasL–TRAIL pathway, or NK/CD8+ T cells. (A–C) Mice were treated as described in Fig. 2. Results depict Foxp3 expression by CD4+CD45.1+ Trp1 cells in complement C3−/− mice (A), mice treated with blocking α-FasL, and α-TRAIL antibodies (B), or mice treated with depleting α-CD8 and α-NK1.1 antibodies (C). Experiments were repeated two times.
Figure 7.
T reg cell depletion is mediated largely by FcγRIV. (A and B) Wild-type and γ−/− mice were challenged with B16-BL6 and treated as described in Fig. 2. Absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 (A) and CD4+CD45.1− endogenous polyclonal T cells (B). n = 9–10 mice per group. (C) SPR analysis of α–CTLA-4 clone 9H10 binding to FcγRI, FcγRIII, and FcγRIV. (D and E) Wild-type, FcγRIII−/−, and FcγRIV−/− mice were challenged with B16-BL6 and treated as described in Fig. 2. Absolute cell numbers and Foxp3 expression by intratumoral CD4+CD45.1+ Trp1 (D) and CD4+CD45.1− endogenous polyclonal T cells (E). (F) SPR analysis of three different α–CTLA-4 clones (4F10, 9D9, 9H10) binding to FcγRIV. n = 7–10 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were repeated three times and pooled.
Figure 8.
Phagocytic cells expressing high levels of FcγRIV are enriched in the tumor microenvironment. (A–E) Mice were treated as described in Fig. 2. (A) Representative FcγRIV expression by intratumoral CD11b+, NK1.1+, and CD3+ cells. (B) Representative CD11c, I-A, Ly6C, and Ly6G expression by intratumoral CD45.2+CD11b+FcγRIV+ cells. (C) CD3 and CD11b expression on CD45.2+ cells in the lymph node and tumor. n = 10 mice per group. (D) Iba1 and Foxp3 expression in the lymph and tumor of GVAX-treated mice. Bar, 100 µm. (E) FcγRIV expression by CD11b+ cells in the lymph node and tumor. The shaded histogram represents FcγRIV expression by CD11b+ cells from FcγRIV−/− mice. n = 10 mice per group. *, P < 0.05; ***, P < 0.001. Experiments were repeated two times and pooled.
Figure 9.
Tumor-infiltrating T reg cells express high levels of surface CTLA-4. (A and B) Surface CTLA-4 expression by CD4+CD45.1+ Trp1 (A) and CD4+CD45.1− endogenous polyclonal T cells (B) in the tumor and lymph node of mice treated with GVAX (as described in Fig. 2). The shaded histogram represents CTLA-4-huTg mice. n = 11 mice per group. (C) Quantification of surface CTLA-4 expression on CD4+Foxp3+ polyclonal T reg cell in the lymph node and tumor after treatment with GVAX or GVAX+α–CTLA-4. Mice were treated with GVAX or GVAX+α–CTLA-4 (clone 9H10) as described in Fig. 2 and sacrificed on day 10. Surface CTLA-4 expression on endogenous CD4+Foxp3+ T reg cells was detected by staining with α–CTLA-4 clone 4F10, which is not cross-blocked by clone 9H10. n = 11–14 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Experiments were repeated two times and pooled.
Figure 10.
α–CTLA-4 therapy does not increase the intratumoral T eff/T reg cell ratio in FcγRIV−/− mice, and fails to elicit tumor protection. (A and B) The T eff/T reg cell ratio from CD4+CD45.1+ Trp1 (A) and CD4+CD45.1− endogenous polyclonal T cells (B) in the tumor. n = 9–10 mice per group. (C) Survival of wild-type and FcγRIV−/− mice treated with GVAX or GVAX+α–CTLA-4, as described in Fig. 1 A. n = 10–11 mice per group. *, P < 0.05; ***, P < 0.001. Experiments were repeated two times and pooled.
Similar articles
- Checkpoint blockade immunotherapy enhances the frequency and effector function of murine tumor-infiltrating T cells but does not alter TCRβ diversity.
Kuehm LM, Wolf K, Zahour J, DiPaolo RJ, Teague RM. Kuehm LM, et al. Cancer Immunol Immunother. 2019 Jul;68(7):1095-1106. doi: 10.1007/s00262-019-02346-4. Epub 2019 May 18. Cancer Immunol Immunother. 2019. PMID: 31104075 Free PMC article. - Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3+ Regulatory T Cells (Tregs) in Human Cancers.
Sharma A, Subudhi SK, Blando J, Scutti J, Vence L, Wargo J, Allison JP, Ribas A, Sharma P. Sharma A, et al. Clin Cancer Res. 2019 Feb 15;25(4):1233-1238. doi: 10.1158/1078-0432.CCR-18-0762. Epub 2018 Jul 27. Clin Cancer Res. 2019. PMID: 30054281 Free PMC article. - Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors.
Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Duraiswamy J, et al. Cancer Res. 2013 Jun 15;73(12):3591-603. doi: 10.1158/0008-5472.CAN-12-4100. Epub 2013 Apr 30. Cancer Res. 2013. PMID: 23633484 Free PMC article. - Targeting cytotoxic lymphocyte antigen 4 (CTLA-4) in breast cancer.
Jama M, Tabana Y, Barakat KH. Jama M, et al. Eur J Med Res. 2024 Jul 2;29(1):353. doi: 10.1186/s40001-024-01901-9. Eur J Med Res. 2024. PMID: 38956700 Free PMC article. Review. - A Systematic Review of Immunotherapy in Urologic Cancer: Evolving Roles for Targeting of CTLA-4, PD-1/PD-L1, and HLA-G.
Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. Carosella ED, et al. Eur Urol. 2015 Aug;68(2):267-79. doi: 10.1016/j.eururo.2015.02.032. Epub 2015 Mar 29. Eur Urol. 2015. PMID: 25824720 Review.
Cited by
- Advances in immunotherapy for hepatitis B virus associated hepatocellular carcinoma patients.
Cao WH, Zhang YQ, Li XX, Zhang ZY, Li MH. Cao WH, et al. World J Hepatol. 2024 Oct 27;16(10):1158-1168. doi: 10.4254/wjh.v16.i10.1158. World J Hepatol. 2024. PMID: 39474576 Free PMC article. Review. - Differentiation fate of a stem-like CD4 T cell controls immunity to cancer.
Cardenas MA, Prokhnevska N, Sobierajska E, Gregorova P, Medina CB, Valanparambil RM, Greenwald R, DelBalzo L, Bilen MA, Joshi SS, Narayan VM, Master VA, Sanda MG, Kissick HT. Cardenas MA, et al. Nature. 2024 Oct 23. doi: 10.1038/s41586-024-08076-7. Online ahead of print. Nature. 2024. PMID: 39443797 - Single-cell landscape of bronchoalveolar immune cells in patients with immune checkpoint inhibitor-related pneumonitis.
Zhang Z, Zhang L, Wang K, Xie T, Zhang X, Yu W, Li Y, Shen L, Li R, Peng Z. Zhang Z, et al. NPJ Precis Oncol. 2024 Oct 5;8(1):226. doi: 10.1038/s41698-024-00715-6. NPJ Precis Oncol. 2024. PMID: 39369126 Free PMC article. - Combining RAS(ON) G12C-selective inhibitor with SHP2 inhibition sensitises lung tumours to immune checkpoint blockade.
Anastasiou P, Moore C, Rana S, Tomaschko M, Pillsbury CE, de Castro A, Boumelha J, Mugarza E, de Carné Trécesson S, Mikolajczak A, Blaj C, Goldstone R, Smith JAM, Quintana E, Molina-Arcas M, Downward J. Anastasiou P, et al. Nat Commun. 2024 Sep 25;15(1):8146. doi: 10.1038/s41467-024-52324-3. Nat Commun. 2024. PMID: 39322643 Free PMC article. - Neoadjuvant androgen deprivation therapy with or without Fc-enhanced non-fucosylated anti-CTLA-4 (BMS-986218) in high risk localized prostate cancer: a randomized phase 1 trial.
Ager CR, Obradovic A, McCann P, Chaimowitz M, Wang ALE, Shaikh N, Shah P, Pan S, Laplaca CJ, Virk RK, Hill JC, Jugler C, DeFranco G, Bhattacharya N, Scher HI, DeCastro GJ, Anderson CB, McKiernan JM, Spina CS, Stein MN, Runcie K, Drake CG, Califano A, Dallos MC. Ager CR, et al. medRxiv [Preprint]. 2024 Sep 11:2024.09.09.24313308. doi: 10.1101/2024.09.09.24313308. medRxiv. 2024. PMID: 39314954 Free PMC article. Preprint.
References
- Antony P.A., Piccirillo C.A., Akpinarli A., Finkelstein S.E., Speiss P.J., Surman D.R., Palmer D.C., Chan C.-C., Klebanoff C.A., Overwijk W.W., et al. 2005. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174:2591–2601 - PMC - PubMed
- Benigni A., Tomasoni S., Turka L.A., Longaretti L., Zentilin L., Mister M., Pezzotta A., Azzollini N., Noris M., Conti S., et al. 2006. Adeno-associated virus-mediated CTLA4Ig gene transfer protects MHC-mismatched renal allografts from chronic rejection. J. Am. Soc. Nephrol. 17:1665–1672 10.1681/ASN.2006010090 - DOI - PubMed
- Chen H., Liakou C.I., Kamat A., Pettaway C., Ward J.F., Tang D.N., Sun J., Jungbluth A.A., Troncoso P., Logothetis C., Sharma P. 2009. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc. Natl. Acad. Sci. USA. 106:2729–2734 10.1073/pnas.0813175106 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases