Loss of PPARγ expression in mammary secretory epithelial cells creates a pro-breast tumorigenic environment - PubMed (original) (raw)
. 2014 Mar 1;134(5):1055-66.
doi: 10.1002/ijc.28432. Epub 2013 Sep 19.
Affiliations
- PMID: 23934545
- PMCID: PMC4233966
- DOI: 10.1002/ijc.28432
Loss of PPARγ expression in mammary secretory epithelial cells creates a pro-breast tumorigenic environment
Anthony J Apostoli et al. Int J Cancer. 2014.
Abstract
Breast cancer is the leading cause of new cancer diagnoses among women. Using peroxisome proliferator-activated receptor (PPAR)γ((+/-)) mice, we showed normal expression of PPARγ was critical to stop 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast tumorigenesis. PPARγ is expressed in many breast cell types including mammary secretory epithelial (MSE) cells. MSEs proliferate as required during pregnancy, and undergo apoptosis or reversible transdifferentiation during involution once lactation is complete. Thus, MSE-specific loss of PPARγ was hypothesized to enhance DMBA-mediated breast tumorigenesis. To test this, MSE cell-specific PPARγ knockout (PPARγ-MSE KO) and control (PPARγ-WT) mice were generated, mated and allowed to nurse for three days. One week after involution, dams were treated with DMBA to initiate breast tumors, and randomized on week 7 to continue receiving a normal chow diet (DMBA Only: PPARγ-WT, n = 15; PPARγ-MSE KO, n = 25) or one supplemented with a PPARγ activating drug (DMBA + ROSI: PPARγ-WT, n = 17; PPARγ-MSE KO, n = 24), and monitored for changes in breast tumor outcomes. PPARγ-MSE KOs had significantly lower overall survival and decreased mammary tumor latency as compared to PPARγ-WT controls. PPARγ activation significantly reduced DMBA-mediated malignant mammary tumor volumes irrespective of genotype. MSE-specific PPARγ loss resulted in decreased mammary gland expression of PTEN and Bax, increased superoxide anion production, and elevated serum eotaxin and RANTES, creating a protumorigenic environment. Moreover, PPARγ activation in MSEs delayed mammary tumor growth in part by down-regulating Cox-1, Cox-2 and cyclin D1. Collectively, these studies highlight a protective role of MSE-specific PPARγ during breast tumorigenesis, and support a novel chemotherapeutic role of PPARγ activation in breast cancer.
Keywords: PPARγ; breast cancer; chemical carcinogenesis; chemotherapy; knockout mouse model; mammary secretory epithelial cells.
© 2013 The Authors. Published by Wiley Periodicals, Inc. on behalf of UICC.
Figures
Figure 1
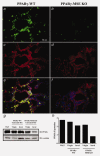
PPARγ protein expression in untreated mammary glands from PPARγ-WT and PPARγ-MSE KO strains. Representative immunofluorescence images illustrates (a and b) PPARγ (FITC; green) and (c and d) β-casein (Alexa Fluor 594; red) expression in lactating glands from both PPARγ-WT and PPARγ-MSE KO mice. An accompanying composite image (e and f) shows PPARγ and β-casein expression together with DAPI-stained nuclei. All photos were taken at ×600. (g) PPARγ expression was analyzed by Western blot in untreated mammary glands (MG) from both strains of virgin mice and mice three days after initiation of involution (Invol). White adipose tissue (WAT) from untreated PPARγ-WT mice was included as a positive control for PPARγ. α-actinin served as a loading control. (h) Densitometry was performed using ImageJ software.
Figure 2
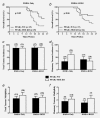
In vivo effects of MSE-specific PPARγ deletion on overall survival, tumor incidence and tumor multiplicity. Female PPARγ-WT and PPARγ-MSE KO mice were treated with DMBA only or DMBA + ROSI, and assessed as described in the Methods section. Overall survival was expressed as the percentage of mice per genotype per treatment group surviving in a given week. Solid lines, PPARγ-WT mice; dashed lines, PPARγ-MSE KO mice; n, number of mice. (a) DMBA only-treated groups; significantly different as compared to respective PPARγ-WT controls, p < 0.05. (b) DMBA + ROSI-treated groups; biologically different as compared to respective PPARγ-WT controls, p = 0.06. (c) Total tumor incidences were calculated as the number of mice with any tumor divided by the total number of mice within a given genotype and treatment group, and expressed as percent + standard error (SE). Black bars, PPARγ-WT mice; White bars, PPARγ-MSE KO mice; number in parentheses, number of mice. (d) Mammary tumor incidences were similarly calculated based on the number of mice with mammary tumors within a given genotype and treatment group, and expressed as percent + SE. (e) Total tumor multiplicity, expressed as a mean ± SE, was defined as the total number of lesions per tumor-bearing mice for a given genotype and treatment group. (f) Mammary tumor multiplicity was calculated as the number of mammary tumors per mouse afflicted with a breast lesion for a given genotype and treatment group, and expressed as a mean + SE.
Figure 3
In vivo effects of MSE-specific PPARγ deletion on mammary tumor volume and latency. Mammary tumors were measured at necropsy, and volumes calculated using the standard formula (L × _W_2/2) and expressed as mean mm3 for each treatment group (a). Open and closed circles, benign and malignant mammary tumors, respectively from PPARγ-WT mice; Open and closed boxes, benign and malignant mammary tumors, respectively from PPARγ-MSE KO mice; Solid bars represent median values for all mammary tumors; dashed bars represent median values for pooled malignant mammary tumors for a given treatment; number in parentheses, number of tumors; *, significantly different from DMBA only-treated groups, p < 0.05. Latency of mammary tumors is expressed as the percentage of mice with palpable mammary tumors within a given genotype and treatment group for a given week. Solid lines, PPARγ-WT mice; dashed lines, PPARγ-MSE KO mice; n, number of mice. (b) DMBA only-treated groups; significantly different compared to respective PPARγ-WT controls, p < 0.05. (c) DMBA + ROSI-treated groups. n, number of mice.
Figure 4
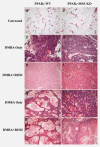
Mammary tumor subtypes among treated PPARγ-WT and PPARγ-MSE KO mice. Representative H&E images of: untreated, involuted mammary glands from (a) PPARγ-WT and (b) PPARγ-MSE KO strains; adenocarcinomas from (c) DMBA only-treated PPARγ-WT and (d) PPARγ-MSE KO mice and (e) DMBA + ROSI-treated PPARγ-WT and (f) PPARγ-MSE KO mice; and squamous cell carcinomas from (g) DMBA-only-treated PPARγ-WT and (h) PPARγ-MSE KO strains and (i) DMBA + ROSI-treated PPARγ-WT and (j) PPARγ-MSE KO strains. All photos were taken at ×100.
Figure 5
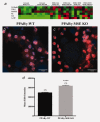
Serum cytokine and in situ superoxide production resulting from MSE-specific PPARγ loss. (a) Heat map illustrating serum cytokine expression profiles for genotypes and treatment groups. Values are Log2 (mean cytokine concentration, pg/ml) with red, black and green indicated high, median and low, respectively. DAPI- and EtBr-stained nuclei (orange) are shown in representative confocal images of lactating mammary glands from (b) untreated PPARγ-WT and (c) PPARγ-MSE KO mice. Both photos were taken at ×600. (d) EtBr fluorescence intensity, expressed as mean + SE, was measured using Metamorph imaging software. ****, significantly different from PPARγ-WT controls, p ≤ 0.0001.
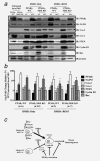
Figure 6
Molecular analysis from untreated mammary glands and mammary tumors from treated PPARγ-WT and PPARγ-MSE KO mice. Representative expression changes within untreated mammary glands (MG) and in vivo generated mammary tumors were analyzed by (a) Western blot as described in the Methods section. PPARγ, 5-LPO, Cox-2, Cox-1, PTEN, cyclin D1 and Bax protein levels were analyzed in untreated, involuted (Invol) MG from PPARγ-WT and PPARγ-MSE KO mice, as well as representative breast tumor subtypes from both strains of mice across both treatment groups. Mammary tumor subtypes include adenocarcinomas (AC), and squamous cell carcinomas (SCC). β-actin served as the loading control. (b) Densitometry was performed on malignant tumors using ImageJ software, and expressed as mean ± SD. Fold changes are relative to involuted mammary tissue from untreated WT. *, Significantly different from DMBA Only-treated groups, p ≤ 0.05. (c) Summary of proposed antibreast tumor MSE-specific PPARγ signaling. Deletion of PPARγ in MSE cells promotes a pro-tumorigenic environment, characterized by increased superoxide production, elevated eotaxin and RANTES circulation, as well as reduced PTEN and Bax protein expression in involuted mammary glands of PPARγ-MSE KO mice. Thus, upon exposure to DMBA, PPARγ-MSE KOs are more susceptible to breast tumorigenesis as compared to similarly treated PPARγ-WT mice. PPARγ activation protects against these effects, albeit more so in PPARγ-WT mice, at least in part by suppressing Cox-1, Cox-2 and Cyclin D1.
Similar articles
- Opposing roles for mammary epithelial-specific PPARγ signaling and activation during breast tumour progression.
Apostoli AJ, Roche JM, Schneider MM, SenGupta SK, Di Lena MA, Rubino RE, Peterson NT, Nicol CJ. Apostoli AJ, et al. Mol Cancer. 2015 Apr 15;14:85. doi: 10.1186/s12943-015-0347-8. Mol Cancer. 2015. PMID: 25889730 Free PMC article. - Stromal adipocyte PPARγ protects against breast tumorigenesis.
Skelhorne-Gross G, Reid AL, Apostoli AJ, Di Lena MA, Rubino RE, Peterson NT, Schneider M, SenGupta SK, Gonzalez FJ, Nicol CJ. Skelhorne-Gross G, et al. Carcinogenesis. 2012 Jul;33(7):1412-20. doi: 10.1093/carcin/bgs173. Epub 2012 May 11. Carcinogenesis. 2012. PMID: 22581835 - Androgen resistance in female mice increases susceptibility to DMBA-induced mammary tumors.
Simanainen U, Gao YR, Walters KA, Watson G, Desai R, Jimenez M, Handelsman DJ. Simanainen U, et al. Horm Cancer. 2012 Jun;3(3):113-24. doi: 10.1007/s12672-012-0107-9. Horm Cancer. 2012. PMID: 22370991 Free PMC article. - Differentiation of the mammary gland and susceptibility to carcinogenesis.
Russo J, Tay LK, Russo IH. Russo J, et al. Breast Cancer Res Treat. 1982;2(1):5-73. doi: 10.1007/BF01805718. Breast Cancer Res Treat. 1982. PMID: 6216933 Review. - Ductal barriers in mammary epithelium.
Owens MB, Hill AD, Hopkins AM. Owens MB, et al. Tissue Barriers. 2013 Oct 1;1(4):e25933. doi: 10.4161/tisb.25933. Epub 2013 Aug 9. Tissue Barriers. 2013. PMID: 24665412 Free PMC article. Review.
Cited by
- Opposing roles for mammary epithelial-specific PPARγ signaling and activation during breast tumour progression.
Apostoli AJ, Roche JM, Schneider MM, SenGupta SK, Di Lena MA, Rubino RE, Peterson NT, Nicol CJ. Apostoli AJ, et al. Mol Cancer. 2015 Apr 15;14:85. doi: 10.1186/s12943-015-0347-8. Mol Cancer. 2015. PMID: 25889730 Free PMC article. - Paternal malnutrition programs breast cancer risk and tumor metabolism in offspring.
da Cruz RS, Carney EJ, Clarke J, Cao H, Cruz MI, Benitez C, Jin L, Fu Y, Cheng Z, Wang Y, de Assis S. da Cruz RS, et al. Breast Cancer Res. 2018 Aug 30;20(1):99. doi: 10.1186/s13058-018-1034-7. Breast Cancer Res. 2018. PMID: 30165877 Free PMC article. - MLAA-34 knockdown shows enhanced antitumor activity via JAK2/STAT3 signaling pathway in acute monocytic leukemia.
Lei B, Qian L, Zhang Y, Chen Y, Gao M, Shah W, Cao X, Zhang P, Zhao W, Liu J, Wang J, Ma X, Yang Y, Meng X, Cai F, Xu Y, Luo J, Wang B, Zhang Y, He A, Zhang W. Lei B, et al. J Cancer. 2020 Sep 30;11(23):6768-6781. doi: 10.7150/jca.46670. eCollection 2020. J Cancer. 2020. PMID: 33123268 Free PMC article. - Peroxisome Proliferator-Activated Receptors and the Hallmarks of Cancer.
Wagner N, Wagner KD. Wagner N, et al. Cells. 2022 Aug 5;11(15):2432. doi: 10.3390/cells11152432. Cells. 2022. PMID: 35954274 Free PMC article. Review. - Study on the effects of the different polar group of EPA-enriched phospholipids on the proliferation and apoptosis in 95D cells.
Guo Y, Zhao Q, Tian Y, Liu Y, Yan Z, Xue C, Wang J. Guo Y, et al. Mar Life Sci Technol. 2021 Jun 7;3(4):519-528. doi: 10.1007/s42995-021-00097-9. eCollection 2021 Nov. Mar Life Sci Technol. 2021. PMID: 37073266 Free PMC article.
References
- American_Cancer_Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2012.
- Canadian_Cancer_Society. Canadian Cancer Statistics. Toronto, ON: Canadian Cancer Society; 2012.
- Lambe M, Hsieh C, Trichopoulos D, et al. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331:5–9. - PubMed
- Lehrke M, Lazar MA. The many faces of PPARγamma. Cell. 2005;123:993–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials