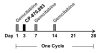A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma - PubMed (original) (raw)
Clinical Trial
A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma
Gregory L Beatty et al. Clin Cancer Res. 2013.
Abstract
Purpose: This phase I study investigated the maximum-tolerated dose (MTD), safety, pharmacodynamics, immunologic correlatives, and antitumor activity of CP-870,893, an agonist CD40 antibody, when administered in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma (PDA).
Experimental design: Twenty-two patients with chemotherapy-naïve advanced PDA were treated with 1,000 mg/m(2) gemcitabine once weekly for three weeks with infusion of CP-870,893 at 0.1 or 0.2 mg/kg on day three of each 28-day cycle.
Results: CP-870,893 was well-tolerated; one dose-limiting toxicity (grade 4, cerebrovascular accident) occurred at the 0.2 mg/kg dose level, which was estimated as the MTD. The most common adverse event was cytokine release syndrome (grade 1 to 2). CP-870,893 infusion triggered immune activation marked by an increase in inflammatory cytokines, an increase in B-cell expression of costimulatory molecules, and a transient depletion of B cells. Four patients achieved a partial response (PR). 2-[(18)F]fluoro-2-deoxy-d-glucose-positron emission tomography/computed tomography (FDG-PET/CT) showed more than 25% decrease in FDG uptake within primary pancreatic lesions in six of eight patients; however, responses observed in metastatic lesions were heterogeneous, with some lesions responding with complete loss of FDG uptake, whereas other lesions in the same patient failed to respond. Improved overall survival correlated with a decrease in FDG uptake in hepatic lesions (R = -0.929; P = 0.007).
Conclusions: CP-870,893 in combination with gemcitabine was well-tolerated and associated with antitumor activity in patients with PDA. Changes in FDG uptake detected on PET/CT imaging provide insight into therapeutic benefit. Phase II studies are warranted.
©2013 AACR.
Figures
Figure 1
Treatment schema. Gemcitabine (1000 mg/m2) was infused on days 1, 8, and 15 of each 28 day cycle with CP-870,893 administered once during each treatment cycle on day 3.
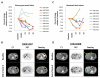
Figure 2
FDG-PET/CT imaging of (A-B) primary pancreatic tumors and (C-D) metastatic hepatic lesions. (A, C) Change relative to baseline in MVPmean as a measure of metabolic activity. (B, D) CT, FDG-PET and fused PET/CT images (baseline vs. end of cycle 4) showing (B) a responding primary pancreatic lesion (green arrow) and (D) a mixed response in liver with representative new or progressive lesions (red arrowheads), stable lesions (yellow arrowheads), and responding lesions (green arrows). Also seen is normal FDG excretion in kidneys and ureters.
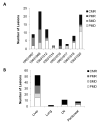
Figure 3
The response of individual lesions measured by changes in lesional MVPmean from baseline to end of cycle 2, shown for (A) each patient and (B) for each metastatic site (all patient lesions pooled) including liver, lung, lymph node (LN), and peritoneal cavity. Changes in MVPmean were classified as complete metabolic response (CMR) indicating disappearance of lesion; partial metabolic response (PMR) indicating >25% reduction in MVPmean; progressive metabolic disease (PMD) indicating >25% increase in MVPmean or new lesion; and stable metabolic disease (SMD) indicating insufficient decrease to qualify for PMR and insufficient increase to qualify for PMD.
Figure 4
Biological correlatives for overall survival after treatment with CP-870,893 in combination with gemcitabine. (A) Median overall survival and progression free survival for patients (n=21) receiving at least one dose of CP-870,893. Relationship between (B) percent change in CA19-9 at end of one cycle of therapy compared to baseline (R = −0.579; p = 0.049), (C) overall survival and tumor response determined by RECIST 1.0 at completion of two cycles of therapy (R = −0.652; p = 0.002), and (D) percent change in total MVPmean for all lesions, primary pancreatic lesion, and all hepatic lesions after two weeks of therapy compared to baseline (R = −0.929; p = 0.007). Best overall tumor response measured by RECIST 1.0 is indicated in (B), (C), and (D) using open circles (partial response), grey circles (stable disease), and black circles (progressive disease).
Comment in
- Harnessing immune responses in the tumor microenvironment: all signals needed.
Le DT, Jaffee EM. Le DT, et al. Clin Cancer Res. 2013 Nov 15;19(22):6061-3. doi: 10.1158/1078-0432.CCR-13-2424. Epub 2013 Oct 4. Clin Cancer Res. 2013. PMID: 24097857 Free PMC article.
Similar articles
- CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans.
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH. Beatty GL, et al. Science. 2011 Mar 25;331(6024):1612-6. doi: 10.1126/science.1198443. Science. 2011. PMID: 21436454 Free PMC article. Clinical Trial. - A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer.
Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH Jr, Bagalà C, Colombi F, Cagnazzo C, Gioeni L, Wang E, Huang B, Fly KD, Leone F. Aglietta M, et al. Ann Oncol. 2014 Sep;25(9):1750-1755. doi: 10.1093/annonc/mdu205. Epub 2014 Jun 6. Ann Oncol. 2014. PMID: 24907635 Clinical Trial. - Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial.
Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M. Von Hoff DD, et al. J Clin Oncol. 2011 Dec 1;29(34):4548-54. doi: 10.1200/JCO.2011.36.5742. Epub 2011 Oct 3. J Clin Oncol. 2011. PMID: 21969517 Free PMC article. Clinical Trial. - [Gemcitabine and pancreatic cancer].
Prost P, Ychou M, Azria D. Prost P, et al. Bull Cancer. 2002 Aug;89 Spec No:S91-5. Bull Cancer. 2002. PMID: 12449037 Review. French. - Emerging drugs in pancreatic cancer.
Ducreux M, Boige V, Malka D. Ducreux M, et al. Expert Opin Emerg Drugs. 2004 May;9(1):73-89. doi: 10.1517/eoed.9.1.73.32946. Expert Opin Emerg Drugs. 2004. PMID: 15155137 Review.
Cited by
- Cisplatin and gemcitabine exert opposite effects on immunotherapy with PD-1 antibody in K-ras-driven cancer.
Glorieux C, Xia X, You X, Wang Z, Han Y, Yang J, Noppe G, Meester C, Ling J, Robert A, Zhang H, Li SP, Wang H, Chiao PJ, Zhang L, Li X, Huang P. Glorieux C, et al. J Adv Res. 2022 Sep;40:109-124. doi: 10.1016/j.jare.2021.12.005. Epub 2021 Dec 21. J Adv Res. 2022. PMID: 36100320 Free PMC article. - Definitive activation of endogenous antitumor immunity by repetitive cycles of cyclophosphamide with interspersed Toll-like receptor agonists.
Manrique SZ, Dominguez AL, Mirza N, Spencer CD, Bradley JM, Finke JH, Lee JJ, Pease LR, Gendler SJ, Cohen PA. Manrique SZ, et al. Oncotarget. 2016 Jul 12;7(28):42919-42942. doi: 10.18632/oncotarget.10190. Oncotarget. 2016. PMID: 27341020 Free PMC article. - The interaction of anticancer therapies with tumor-associated macrophages.
Mantovani A, Allavena P. Mantovani A, et al. J Exp Med. 2015 Apr 6;212(4):435-45. doi: 10.1084/jem.20150295. Epub 2015 Mar 9. J Exp Med. 2015. PMID: 25753580 Free PMC article. Review. - Immune checkpoint and inflammation as therapeutic targets in pancreatic carcinoma.
Kimbara S, Kondo S. Kimbara S, et al. World J Gastroenterol. 2016 Sep 7;22(33):7440-52. doi: 10.3748/wjg.v22.i33.7440. World J Gastroenterol. 2016. PMID: 27672267 Free PMC article. Review. - The Role of Myeloid Cells in Hepatotoxicity Related to Cancer Immunotherapy.
Gudd CLC, Possamai LA. Gudd CLC, et al. Cancers (Basel). 2022 Apr 10;14(8):1913. doi: 10.3390/cancers14081913. Cancers (Basel). 2022. PMID: 35454819 Free PMC article. Review.
References
- American Cancer Society Cancer facts and figures. 2012.
- Hidalgo M. Pancreatic cancer. The New England journal of medicine. 2010;362:1605–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000005/TR/NCATS NIH HHS/United States
- K08 CA138907/CA/NCI NIH HHS/United States
- R01 CA158186/CA/NCI NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- R01 CA169123/CA/NCI NIH HHS/United States
- K08 CA138907-02/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous