Type 2 innate lymphoid cells control eosinophil homeostasis - PubMed (original) (raw)
. 2013 Oct 10;502(7470):245-8.
doi: 10.1038/nature12526. Epub 2013 Sep 15.
Affiliations
- PMID: 24037376
- PMCID: PMC3795960
- DOI: 10.1038/nature12526
Type 2 innate lymphoid cells control eosinophil homeostasis
Jesse C Nussbaum et al. Nature. 2013.
Abstract
Eosinophils are specialized myeloid cells associated with allergy and helminth infections. Blood eosinophils demonstrate circadian cycling, as described over 80 years ago, and are abundant in the healthy gastrointestinal tract. Although a cytokine, interleukin (IL)-5, and chemokines such as eotaxins mediate eosinophil development and survival, and tissue recruitment, respectively, the processes underlying the basal regulation of these signals remain unknown. Here we show that serum IL-5 levels are maintained by long-lived type 2 innate lymphoid cells (ILC2) resident in peripheral tissues. ILC2 cells secrete IL-5 constitutively and are induced to co-express IL-13 during type 2 inflammation, resulting in localized eotaxin production and eosinophil accumulation. In the small intestine where eosinophils and eotaxin are constitutive, ILC2 cells co-express IL-5 and IL-13; this co-expression is enhanced after caloric intake. The circadian synchronizer vasoactive intestinal peptide also stimulates ILC2 cells through the VPAC2 receptor to release IL-5, linking eosinophil levels with metabolic cycling. Tissue ILC2 cells regulate basal eosinophilopoiesis and tissue eosinophil accumulation through constitutive and stimulated cytokine expression, and this dissociated regulation can be tuned by nutrient intake and central circadian rhythms.
Figures
Figure 1. Innate cells produce IL-5 in tissues at rest
a, Schematic of targeting construct. b-c, Flow cytometry of tissues, previously gated on CD45+CD90.2+ cells in wild-type and R5/+ (b) or CD90.2+ cells in R5/R5 (c) naïve mice. d, Serum IL-5. Data representative of two independent experiments with two mice per group (b-d) or pooled from three independent experiments for 7 (wild-type), 4 (Red5), or 8 (others) mice per group (c). LN, lymph nodes; ND, none detected; NS not significant; *, p < 0.05.
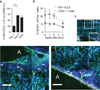
Figure 2. ILC2 expand after birth and persist in collagen-rich structures
a, Percent of lung Lin-CD90.2+ cells R5+T1/ST2 on day 1, day 8, or week 8. b, Percent BrdU+ of R5+ ILC2 and total CD4+ cells in lung after four weeks BrdU. c, Representative multiphoton images of tdTomato fluorescence (red) in naïve R5/R5 actin-CFP mice; CFP and autofluorescence in blue and green, respectively. A=airway. V=vasculature. Collagen second harmonic appears blue. Scale bars 100 µm. Data pooled from three independent experiments for 5 (Day 1), 6 (Day 8), or 4 (Adult) mice per group (a); or pooled from two independent experiments for 5 (week 0), 6 (week 1), or 3 (others) mice per group (b), represented as mean +/− SEM. Images represent 8 regions taken from two mice. Lin, Lineage markers (B220, CD5, CD11b, CD11c, Ly6G, FcεRI, and NK1.1); ***, p < 0.01 by Student’s t test.
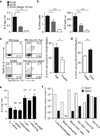
Figure 3. IL-5 and IL-13 co-expression in lung ILC2
a, Lung IL-5 and IL-13 reporter expression before and after infection. b, Flow cytometry of CD90.2+ lung cells and percent with ILC2 surface markers (CD90.2 and either KRLG1, T1/ST2 or CD25) at rest. c, ILC2 (left lung) and CCL11 concentration (right lung) after IL-2, IL-33, and IL-13 treatment. Data representative of three independent experiments with 4 (naïve R5+S13+), 5 (infected R5+S13+), or 2 (others) mice per group (a), pooled from three independent experiments for 6 (R5/R5 bone marrow and spleen) or 9 (all others) mice per group (b), or pooled from two independent experiments for 8 (R5/R5 + IL-2/IL-33), 5 (R5/R5 Deleter + IL-2/IL-33/IL-13), or 3 (others) mice per group (c). Represented as mean +/− SEM. huCD4, human CD4; BM, bone marrow; NS, not significant; *, p < 0.05; ***, p < 0.001 by Student’s t test.
Figure 4. ILC2 respond to circadian and metabolic cues
a-b, Serum IL-5 and blood eosinophils at 10:00, 22:00 or at 10:00 after fasting. c-d, Flow cytometry of small intestine ILC2 (Lin-CD127+ICOS+) and percent of R5-hi ILC2 expressing S13 at 8:00 in mice on nighttime (black) or daytime (white) feeding (c) or in fasted mice given food (black) or water (white) by oral gavage (d). e, Supernatant IL-5 from intestinal Lin-CD45+KLRG1+ ILC2 cultured in IL-7 alone or with indicated reagents. f, Expression of Vpac1 and Vpac2 in sorted cells, relative to Rps17. Data pooled from independent experiments for 19 (AM), 6 (PM), or 5 (fasted) mice per group (a); 7 (AM), 4 (PM), or 8 (fasted) mice per group (b); 8 mice per group (c); 6 mice per group (d); or pooled averages of duplicate cultures from 6 (IL-7 alone, + VIP, + VPAC2 agonist) or 3 (all others) cell sorts from independent mice (e), or representative of two experiments of independent cell sorts (f). Represented as mean +/− SEM. Lin, Lineage markers (B220, CD11b, CD11c, Ly6G, FcεRI, and NK1.1); Rps17, 40S ribosomal protein S17; NS, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001 by Student’s t test.
Comment in
- Keeping eosinophils on time--ILC2 it!
Bordon Y. Bordon Y. Nat Rev Immunol. 2013 Nov;13(11):774-5. doi: 10.1038/nri3549. Epub 2013 Oct 7. Nat Rev Immunol. 2013. PMID: 24096334 No abstract available.
Similar articles
- Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection.
Gorski SA, Hahn YS, Braciale TJ. Gorski SA, et al. PLoS Pathog. 2013 Sep;9(9):e1003615. doi: 10.1371/journal.ppat.1003615. Epub 2013 Sep 19. PLoS Pathog. 2013. PMID: 24068930 Free PMC article. - The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation.
Klose CSN, Mahlakõiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, Shen X, Kostenis E, König GM, Senda T, Carpenter D, Farber DL, Artis D. Klose CSN, et al. Nature. 2017 Sep 14;549(7671):282-286. doi: 10.1038/nature23676. Epub 2017 Sep 6. Nature. 2017. PMID: 28869965 Free PMC article. - Nematode-Infected Mice Acquire Resistance to Subsequent Infection With Unrelated Nematode by Inducing Highly Responsive Group 2 Innate Lymphoid Cells in the Lung.
Yasuda K, Adachi T, Koida A, Nakanishi K. Yasuda K, et al. Front Immunol. 2018 Sep 19;9:2132. doi: 10.3389/fimmu.2018.02132. eCollection 2018. Front Immunol. 2018. PMID: 30283458 Free PMC article. - Gastrointestinal eosinophils.
Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Rothenberg ME, et al. Immunol Rev. 2001 Feb;179:139-55. doi: 10.1034/j.1600-065x.2001.790114.x. Immunol Rev. 2001. PMID: 11292017 Review. - Elemental signals regulating eosinophil accumulation in the lung.
Foster PS, Mould AW, Yang M, Mackenzie J, Mattes J, Hogan SP, Mahalingam S, Mckenzie AN, Rothenberg ME, Young IG, Matthaei KI, Webb DC. Foster PS, et al. Immunol Rev. 2001 Feb;179:173-81. doi: 10.1034/j.1600-065x.2001.790117.x. Immunol Rev. 2001. PMID: 11292021 Review.
Cited by
- Chronic tissue inflammation and metabolic disease.
Lee YS, Olefsky J. Lee YS, et al. Genes Dev. 2021 Mar 1;35(5-6):307-328. doi: 10.1101/gad.346312.120. Genes Dev. 2021. PMID: 33649162 Free PMC article. Review. - IL-33-driven ILC2/eosinophil axis in fat is induced by sympathetic tone and suppressed by obesity.
Ding X, Luo Y, Zhang X, Zheng H, Yang X, Yang X, Liu M. Ding X, et al. J Endocrinol. 2016 Oct;231(1):35-48. doi: 10.1530/JOE-16-0229. J Endocrinol. 2016. PMID: 27562191 Free PMC article. - Cytokine response during non-cerebral and cerebral malaria: evidence of a failure to control inflammation as a cause of death in African adults.
Dieye Y, Mbengue B, Dagamajalu S, Fall MM, Loke MF, Nguer CM, Thiam A, Vadivelu J, Dieye A. Dieye Y, et al. PeerJ. 2016 May 2;4:e1965. doi: 10.7717/peerj.1965. eCollection 2016. PeerJ. 2016. PMID: 27168977 Free PMC article. - Collaborative interactions between type 2 innate lymphoid cells and antigen-specific CD4+ Th2 cells exacerbate murine allergic airway diseases with prominent eosinophilia.
Liu B, Lee JB, Chen CY, Hershey GK, Wang YH. Liu B, et al. J Immunol. 2015 Apr 15;194(8):3583-93. doi: 10.4049/jimmunol.1400951. Epub 2015 Mar 16. J Immunol. 2015. PMID: 25780046 Free PMC article. - Novel Inhibitory Effect of a Lysophosphatidic Acid 2 Agonist on Allergen-Driven Airway Inflammation.
Knowlden SA, Hillman SE, Chapman TJ, Patil R, Miller DD, Tigyi G, Georas SN. Knowlden SA, et al. Am J Respir Cell Mol Biol. 2016 Mar;54(3):402-9. doi: 10.1165/rcmb.2015-0124OC. Am J Respir Cell Mol Biol. 2016. PMID: 26248018 Free PMC article.
References
- Rothenberg ME, Hogan SP. The Eosinophil. Annual Review of Immunology. 2006;24:147–174. - PubMed
- Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288–294. - PubMed
- Pope S, et al. IL-13 induces eosinophil recruitment into the lung by an IL-5 and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. - PubMed
- Kopf M, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI078869/AI/NIAID NIH HHS/United States
- P01 HL024136/HL/NHLBI NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- T32 AI007334/AI/NIAID NIH HHS/United States
- T32 GM007618/GM/NIGMS NIH HHS/United States
- P01 HL107202/HL/NHLBI NIH HHS/United States
- DP1 AR064158/AR/NIAMS NIH HHS/United States
- R01 AI026918/AI/NIAID NIH HHS/United States
- DK063720/DK/NIDDK NIH HHS/United States
- U19 AI077439/AI/NIAID NIH HHS/United States
- T32 AI007641/AI/NIAID NIH HHS/United States
- T32 HD044331/HD/NICHD NIH HHS/United States
- R37 AI026918/AI/NIAID NIH HHS/United States
- R01 AI030663/AI/NIAID NIH HHS/United States
- AI026918/AI/NIAID NIH HHS/United States
- AI007334/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- AI030663/AI/NIAID NIH HHS/United States
- AI007641/AI/NIAID NIH HHS/United States
- AI078869/AI/NIAID NIH HHS/United States
- HL107202/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials