Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors - PubMed (original) (raw)
. 2013 Nov 15;126(Pt 22):5305-12.
doi: 10.1242/jcs.138578. Epub 2013 Sep 17.
Affiliations
- PMID: 24046449
- PMCID: PMC3828596
- DOI: 10.1242/jcs.138578
Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors
Ryan J Park et al. J Cell Sci. 2013.
Abstract
Dynamin, which is encoded by three genes in mammals, is a GTPase implicated in endocytic membrane fission. Dynamin 1 and 3 are predominantly expressed in brain, whereas dynamin 2 is ubiquitously expressed. With the goal of assessing the impact of the lack of dynamin on cell physiology, we previously generated and characterized dynamin 1 and 2 double knockout (DKO) fibroblasts. These DKO cells were unexpectedly viable in spite of a severe impairment of clathrin-mediated endocytosis. As low-level expression of the dynamin 3 gene in these cells could not be excluded, we have now engineered dynamin 1, 2 and 3 triple KO (TKO) fibroblasts. These cells did not reveal any additional defects beyond what was previously observed in DKO fibroblasts. Surprisingly, although fluid-phase endocytosis and peripheral membrane ruffling were not impaired by the lack of all three dynamins, two structurally similar, widely used dynamin inhibitors, dynasore and Dyngo-4a, robustly inhibited these two processes both in wild-type and TKO cells. Dynamin TKO cells will be useful tools for the further exploration of dynamin-dependent processes and the development of more specific dynamin inhibitors.
Keywords: Actin; Dynamin; Dynasore; Dyngo; Synapse; Synaptic vesicle.
Figures
Fig. 1.
Generation of dynamin 1, 2 and 3 TKO fibroblasts. (A) Immunoblotting with isoform-specific anti-dynamin antibodies of total homogenates of fibroblasts generated from mice with floxed (fl) dynamin alleles as indicated, and also heterozygous for the transgenic expression of Cre-ER. The anti-clathrin heavy chain (clathrin HC) blot is included as a loading control. +OHT indicates lysates derived from cells treated with 4-hydroxytamoxifen to induce Cre-dependent gene recombination. (B) PCR bands demonstrating recombination of dynamin loci and presence of the Cre recombinase gene. The asterisk indicates a PCR fragment of the floxed allele of dynamin 3, which is larger than the WT allele because of the presence of a loxP site.
Fig. 2.
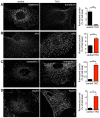
Defects in clathrin-mediated endocytosis and actin distribution in dynamin TKO cells. (A) Impaired internalization of fluorescent transferrin in TKO cells. (B) Increased abundance of endocytic clathrin-coated pits in TKO cells as revealed by immunofluorescence for the α-adaptin subunit of the AP2 clathrin adaptor complex. (C,D) Increased abundance in TKO cells of endophilin 2 (C)- and Arp 2/3 (ARPC2 subunit; D)-positive puncta. These puncta are known to reflect the accumulation of these proteins at the collars of arrested clathrin-coated pits in dynamin-deficient cells (Ferguson et al., 2009). Quantifications of the fluorescence signals for all conditions are shown on the right (5–18 cells per condition). Scale bars: 10 µm.
Fig. 3.
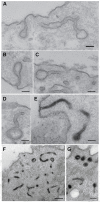
Clathrin-coated pits connected to the plasma membrane by long, narrow tubular necks in dynamin TKO cells. (A–D) Representative EM images from conventionally stained sections. (E–G) Presence of electron dense material in tubular invaginations of the plasma membrane, some of which are capped by a clathrin-coated bud in the plane of the section, following a cytochemical reaction (black reaction product) that selectively labels internal structures continuous with plasma membrane. Scale bars: 100 nm (A–E), 400 nm (F); 200 nm (G).
Fig. 4.
Fluid-phase endocytosis is not impaired in TKO cells, but is inhibited by dynasore or Dyngo-4a in both control and TKO cells. (A) Fluorescence images showing that Alexa-Fluor-488–dextran internalization is not impaired (in fact slightly enhanced) in TKO cells relative to control cells, but is strongly inhibited by dynasore or Dyngo-4a. The periphery of dynasore- and Dyngo-4a-treated cells is outlined by dotted lines. Scale bars: 10 µm. (B) Quantification of the results shown in A; n = 10 cells per condition. DMSO, which was used to dissolve dynasore or Dyngo-4a, was present in control and drug-treated cells at the final concentration of 0.15% (+DMSO), 0.15% (+dynasore), and 0.1% (+Dyngo-4a).
Fig. 5.
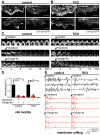
Dynasore and Dyngo-4a inhibit peripheral membrane ruffling in both control and TKO cells. Analysis of the effect of dynasore (80 µM, 37°C) or Dyngo-4a (30 µM, 37°C) on peripheral membrane ruffling in control and TKO cells expressing calponin homology (CH) domain of utrophin (CH-Utr, a live marker of F-actin) fused to GFP (spinning disk confocal microscopy). Fluorescence images of small regions of the cell periphery of control (A) and TKO cells (B) expressing CH-Utr fused to GFP before and after 20 minutes incubation with dynasore (top) or Dyngo-4a (bottom), showing the disrupting effect of these drugs on the ruffles in both control and TKO cells. (C) Time series (120 second intervals) of cropped images of the edge of control (left) and TKO (right) cells showing persistence of membrane ruffles over 20 minutes (top), but their rapid disappearance in response to dynasore (middle) or Dyngo-4a (bottom), but not in response to the DMSO used to solubilize these drugs (top). (D) Quantification of cell motility of control and TKO cells in response to dynasore or Dyngo-4a; n = 3–5 cells per condition. (E) Representative graphs showing the fluorescence intensity changes of three randomly selected points in membrane ruffles of control (left) or TKO (right) cells in the absence and presence of dynasore or Dyngo-4a. Scale bars: 10 µm (A,B); 3 µm (C). DMSO, which was used to dissolve dynasore, was present in control and dynasore-treated cells at the final concentration of 0.15% (DMSO only), 0.15% (dynasore treatment) or 0.1% (Dyngo-4a treatment).
Similar articles
- Herpes Simplex Virus 1 Can Enter Dynamin 1 and 2 Double-Knockout Fibroblasts.
Möckel M, Rahn E, de la Cruz N, Wirtz L, van Lent JWM, Pijlman GP, Knebel-Mörsdorf D. Möckel M, et al. J Virol. 2019 Jul 30;93(16):e00704-19. doi: 10.1128/JVI.00704-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31142668 Free PMC article. - Overlapping role of dynamin isoforms in synaptic vesicle endocytosis.
Raimondi A, Ferguson SM, Lou X, Armbruster M, Paradise S, Giovedi S, Messa M, Kono N, Takasaki J, Cappello V, O'Toole E, Ryan TA, De Camilli P. Raimondi A, et al. Neuron. 2011 Jun 23;70(6):1100-14. doi: 10.1016/j.neuron.2011.04.031. Neuron. 2011. PMID: 21689597 Free PMC article. - Chemical Inhibitors of Dynamin Exert Differential Effects in VEGF Signaling.
Basagiannis D, Zografou S, Goula E, Gkeka D, Kolettas E, Christoforidis S. Basagiannis D, et al. Cells. 2021 Apr 23;10(5):997. doi: 10.3390/cells10050997. Cells. 2021. PMID: 33922806 Free PMC article. - Dynasore - not just a dynamin inhibitor.
Preta G, Cronin JG, Sheldon IM. Preta G, et al. Cell Commun Signal. 2015 Apr 10;13:24. doi: 10.1186/s12964-015-0102-1. Cell Commun Signal. 2015. PMID: 25889964 Free PMC article. Review. - Targeting membrane trafficking in infection prophylaxis: dynamin inhibitors.
Harper CB, Popoff MR, McCluskey A, Robinson PJ, Meunier FA. Harper CB, et al. Trends Cell Biol. 2013 Feb;23(2):90-101. doi: 10.1016/j.tcb.2012.10.007. Epub 2012 Nov 17. Trends Cell Biol. 2013. PMID: 23164733 Review.
Cited by
- SP-R210 (Myo18A) Isoforms as Intrinsic Modulators of Macrophage Priming and Activation.
Yang L, Carrillo M, Wu YM, DiAngelo SL, Silveyra P, Umstead TM, Halstead ES, Davies ML, Hu S, Floros J, McCormack FX, Christensen ND, Chroneos ZC. Yang L, et al. PLoS One. 2015 May 12;10(5):e0126576. doi: 10.1371/journal.pone.0126576. eCollection 2015. PLoS One. 2015. PMID: 25965346 Free PMC article. - Different dynamin blockers interfere with distinct phases of synaptic endocytosis during stimulation in motoneurones.
Linares-Clemente P, Rozas JL, Mircheski J, García-Junco-Clemente P, Martínez-López JA, Nieto-González JL, Vázquez ME, Pintado CO, Fernández-Chacón R. Linares-Clemente P, et al. J Physiol. 2015 Jul 1;593(13):2867-88. doi: 10.1113/JP270112. Epub 2015 Jun 10. J Physiol. 2015. PMID: 25981717 Free PMC article. - Clathrin-independent trafficking of AMPA receptors.
Glebov OO, Tigaret CM, Mellor JR, Henley JM. Glebov OO, et al. J Neurosci. 2015 Mar 25;35(12):4830-6. doi: 10.1523/JNEUROSCI.3571-14.2015. J Neurosci. 2015. PMID: 25810514 Free PMC article. - Dynamin Is Required for Efficient Cytomegalovirus Maturation and Envelopment.
Hasan MH, Davis LE, Bollavarapu RK, Mitra D, Parmar R, Tandon R. Hasan MH, et al. J Virol. 2018 Nov 27;92(24):e01418-18. doi: 10.1128/JVI.01418-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282704 Free PMC article. - Cellular Uptake Evaluation of Amphiphilic Polymer Assemblies: Importance of Interplay between Pharmacological and Genetic Approaches.
Jiang Z, He H, Liu H, Thayumanavan S. Jiang Z, et al. Biomacromolecules. 2019 Dec 9;20(12):4407-4418. doi: 10.1021/acs.biomac.9b01073. Epub 2019 Nov 5. Biomacromolecules. 2019. PMID: 31609589 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30DK045735/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- R37NS036251/NS/NINDS NIH HHS/United States
- R37 NS036251/NS/NINDS NIH HHS/United States
- P30 DA018343/DA/NIDA NIH HHS/United States
- P30DA018343/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous